Abstract
Organ preservation is considered in the management of selected patients with rectal cancer. Complete clinical response observed after neoadjuvant chemoradiation for rectal cancer is one of these cases. Patients who present complete clinical response are candidates to the watch-and-wait approach, when radical surgery is not immediately performed and is offered only to patients in the event of a local relapse. These patients are included in a strict follow-up, and up of 70% of them will never be operated during the follow-up. This strategy is associated with similar oncological outcomes as patients operated on, and the advantage of avoiding the morbidity associated to the radical operation. In this article we will discuss in detail the best candidates for this approach, the protocol itself, and the long-term outcomes.
Keywords: rectal cancer, complete response, neoadjuvant therapy, watch-and-wait
Incorporation of new treatment modalities has significantly increased complexity in the management of rectal cancer. 1 Surgical treatment is still the main pillar in the management of rectal cancer. Interest in different approaches for total mesorectal excision (TME), including standard laparoscopy, and robotic and transanal TME, are increasing rapidly. Not only surgical approaches but also the neoadjuvant therapy and the management after assessment of tumor response are changing. Neoadjuvant chemoradiation therapy (nCRT) may lead to significant tumor regression, ultimately leading to complete pathological response in up to 42% of patients. 2 Assessment of tumor response following nCRT and prior to radical surgery may identify patients with complete clinical response (cCR) who could be managed nonoperatively with a strict follow-up (watch-and-wait [WW] strategy), thus avoiding unnecessary postoperative morbidity with good long-term oncological outcomes and excellent functional results. 3 In addition, close surveillance may allow early detection of local recurrences and salvage alternative with no oncological compromise. 4 In this article, we will discuss the management of rectal cancer following cCR after nCRT.
Rationale for Organ Preservation in Rectal Cancer
Different organ-preserving strategies for the treatment of rectal cancer have gained popularity in the last few years. The main reasons for avoiding a proctectomy include the significant postoperative morbidity, including long-term urinary, and sexual and fecal continence dysfunction, in addition to the requirement for temporary or definitive stomas associated with the procedure. Also, depending on the associated comorbidities and patient's age, postoperative mortality may also be quite significant. 5 Therefore, in selected patients with evidence of complete primary tumor regression, surgical and even nonsurgical approaches have been suggested. 6
The observation that rectal cancers could develop significant tumor regression with reduction in primary tumor size (downsizing), depth of tumor penetration, and even potential nodal sterilization (downstaging) could set the ideal stage for organ-preserving alternatives including local excision of small and superficial residual tumors. 7 In addition, regression of the primary tumor could result in complete disappearance of the tumor in the resected specimen (pathological complete response [pCR]) in some patients. In a subset of these patients, complete disappearance of the primary tumor is already clinically detected prior to surgical resection, referred to as complete clinical responses (cCRs). 8 These patients (cCRs) would constitute ideal candidates to consider organ-preserving strategies including no immediate surgical resection of the area harboring the original cancer. 9 To even consider these approaches, colorectal surgeons have to take into consideration several aspects of the disease, patients, and treatment modalities that may be quite relevant during their clinical decision-making process.
Prediction of Response to nCRT and Intratumoral Heterogeneity
Several studies have attempted to provide a clinically useful tool based on molecular biology features of rectal cancers undergoing nCRT to predict response to treatment upfront. 10 This would allow more precise selection of patients who would benefit the most from CRT, spare patients from potentially unnecessary treatment, and allow identification of ideal candidates for nonoperative management. Unfortunately, however, these studies have failed to provide any clinically relevant information to be implemented into clinical practice so far. First, published gene signatures rarely present specific genes overlapping between them. Second, validation of findings between these signatures in independent cohorts often results in inaccurate identification of complete responders to nCRT. 10 11 12 13 14 15 16
The presence of significant intratumoral heterogeneity may have accounted at least in part for these disappointing results. 16 The coexistence of subpopulations of cancer cells within a single rectal cancer with distinct morphological features and genetic mutations may render single-biopsy samples simply not representative of the entirety of the primary tumor. Therefore, a single biopsy sample from one area of the primary tumor may contain cancer cells that are resistant to nCRT, whereas biopsy taken from other areas may contain cancer cells that are sensitive to nCRT. 15
Baseline Staging and Indications for Neoadjuvant Chemoradiation
Following the results of the German Trial, chemoradiation was considered the preferred initial approach for cT3–4 or cN+ rectal cancers due to the potential benefits in local disease control after radical surgery. 17 18 However, data from the Mercury study suggested that after proper or optimal TME, local recurrence was unlikely to develop for most cT3 cancers, even in the presence of nodal disease (cN1). 19 In this scenario, patients at higher risk for local recurrence, and therefore that would most benefit from nCRT to improve local disease control, would be those with radiological evidence of a positive circumferential margin (cCRM+ ), presence of extramural venous invasion (cEMVI+ ) or at least 3 positive lymph nodes (cN2). In addition, radical surgery after nCRT was shown to result in worse functional outcomes and increased surgical morbidity when compared with surgery alone. 20 21 Altogether, these data suggested that nCRT was to be restricted for high-risk patients (also referred to as the “ugly” tumors) for the development of local recurrence only. Considering that baseline staging features may influence the development of complete response to nCRT, one could expect that very few patients with such advanced disease would do so.
However, the possibility of avoiding radical surgery and its related comorbidities after a cCR raised the issue of offering nCRT to more early stage rectal cancers, particularly for the most distal tumors. Ultimately, patients with cT2N0 or early cT3N0 are more likely to develop a cCR following nCRT and could benefit the most from nCRT if organ preservation is considered. 2 9 22 23 24
Therefore, the use of neoadjuvant nCRT should be considered only for high-risk patients (cCRM + , cEMVI, and cN2) if radical surgery is to be performed, regardless of response to treatment. However, if organ-preserving strategies are an option according to tumor response, nCRT may be offered to most rectal cancers (except for cT1N0). 25 Here tumor location or height may be of significant importance. As it will be discussed in the following sections of the article, clinical assessment including digital rectal examination (DRE) is crucial for the identification and surveillance of cCR, and only baseline cancers accessible to DRE (usually up to 7–8 cm from the anal verge) would be appropriate candidates for organ-preserving strategies without immediate surgery. 1
Neoadjuvant Treatment Options
Specific features of neoadjuvant therapy regimen may ultimately affect the odds of developing a cCR and should be considered in the setting of organ-preserving strategies. Initially, it was thought that long-course CRT was the only strategy that could result in significant rates of complete response, whereas short-course CRT would only rarely have such clinical outcome. However, with the understanding of the influence of time on the development of complete response to therapy, it has been suggested that short-course RT followed by delayed assessment of response may result in similar rates of complete response to the observed after long-course CRT. 2 26 27
The dose of radiation therapy (RT) may also influence the odds of patient with rectal cancer in developing complete response to treatment. Dose escalation studies have demonstrated progressive increase in CR rates with higher doses of RT delivered to the primary tumor. 28 29 In addition to the actual dose delivered, the method of delivery may also affect the development of a CR. Therefore, the combination of external beam or intensity-modulated RT (or external beam RT) with endorectal brachytherapy or even contact RT may further increase total dose of radiation delivered, maximizing the chances of developing cCR and still avoiding major treatment related toxicity. 30 31 32
More recently, a strategy has been suggested to provide neoadjuvant therapy with chemotherapy alone prior to RT in an attempt to avoid the toxic and potential morbidity resulting from RT in these patients. 33 The delivery of chemotherapy alone would allow the control of possible micrometastatic foci of the disease while still providing significant response to the primary tumor in a good proportion of patients. Standard CRT could be restricted to patients showing minimal response to chemotherapy alone, therefore minimizing the amount of patients receiving RT. 34
Finally, combinations of standard CRT and more aggressive chemotherapy regimens have been suggested that include additional cycles of chemotherapy being delivered during the resting period after RT completion in standard CRT regimens (consolidation CRT regimens). One study adding additional cycles of 5-fluorouracil (5FU)-based chemotherapy during the resting period after 54 Gy of RT suggested an increase of CR rates to >50% in patients with T2/T3 rectal cancer. Data from a prospective study using standard CRT followed by progressively higher numbers of FOLFOX cycles during the resting periods after RT completion have demonstrated a significant increase in pCR rates after radical surgery. 25 35
Altogether, these data may suggest that if organ preservation is an option, optimization of RT and chemotherapy should be considered upfront rather than after standard CRT.
Assessment of Tumor Response
Considering that patients may develop significant tumor regression after nCRT that may provide an appropriate setting for an organ-preserving strategy, one issue becomes crucial in this process: assessment of tumor response. However, assessment of tumor response may be quite challenging due to numerous uncertainties including optimal timing and clinical/radiological tools for such purpose.
Assessment of tumor response is also recommended even if an organ-preserving strategy is not being considered. Even if the plan after nCRT is a radical resection, one needs to consider that after nCRT, the surgeon may be facing a considerably different tumor. Knowing this potentially new “anatomy” ahead of time may allow the surgeon to optimize intraoperative surgical strategy and to know in advance what challenges could be anticipated during the procedure. 36 Therefore, the reassessment of tumor response should be performed in all patients.
Timing for the Assessment of Tumor Response
The grade of tumor regression after nCRT appears to be a time-dependent phenomenon. The first randomized trial to consider the effect of different time intervals in the response to CRT was a French study comparing 2 versus 6 weeks from nCRT. In this study, all patients underwent radical surgery after these two time intervals, and patients with 6-week intervals presented significantly more tumor regression after nCRT. 37 Due to this study, a 6-week time interval between nCRT completion and performance of radical surgery has been considered the standard of care for many years. However, retrospective studies consistently reported that patients undergoing radical surgery after 6 to 8 weeks from nCRT were more likely to develop pCR. 38 39 40 41 One of these studies suggested that the rates of pCR after nCRT may keep rising after nCRT for as long as 12 weeks from treatment completion. 39 However, there was a question whether these prolonged intervals from nCRT would result in excessive tissue fibrosis in the area included in the RT field that could lead to increased technical difficulty and postoperative morbidity after radical surgery. One study included patients in nCRT regimens with progressively longer interval periods prior to surgery. Even though this was not a randomized study, patients in different groups were comparable. 23 Curiously, patients undergoing surgery after 12 weeks developed similar postoperative complication rates when compared with the standard 6-week interval. The study then kept on recruiting patients for progressively longer intervals: 6, 12, 18, and 24 weeks between nCRT and surgery. Even though additional systemic chemotherapy has been offered to patients undergoing surgery after longer interval periods, delaying surgical resection to ≥20 weeks resulted in significantly higher pCR rates, with no negative impact on postoperative morbidity. 42 Altogether, these data seem to suggest that the longer you wait, more tumor regression is observed and that longer intervals than 6 to 8 weeks would clearly benefit patients after nCRT. However, another recently published randomized study failed to demonstrate the benefits of longer intervals after nCRT. In this study, patients undergoing 7 weeks developed similar pCR rates to patients undergoing 11-week intervals. Moreover, patients undergoing 11-week intervals developed increased rates of postoperative complications and ended up with worse quality of the resected specimen (quality of the mesorectum), suggesting the detrimental effects of prolonged time after nCRT on fibrotic changes in the surgical and previously irradiated fields. 43
The optimal interval after nCRT remains undetermined, and additional ongoing trials will definitely provide more data to allow us to understand the benefits and risks of using prolonged intervals after treatment. In fact, it may be the case that a single and fixed interval may not be appropriate for all patients. Instead, patients/tumors may respond differently as a function of time to nCRT. Ultimately, responsive tumors may require and actually benefit from prolonged intervals from nCRT, whereas unresponsive tumors may not. It is likely that responsive tumors that are being considered for organ-preserving strategies should have their assessment of response and ultimately surgical strategy decision deferred to longer than 12 weeks ( Fig. 1 ). On the other hand, tumors with little response that still require radical TME may benefit from 6- to 8-week intervals between nCRT completion and radical surgery. 44
Fig. 1.
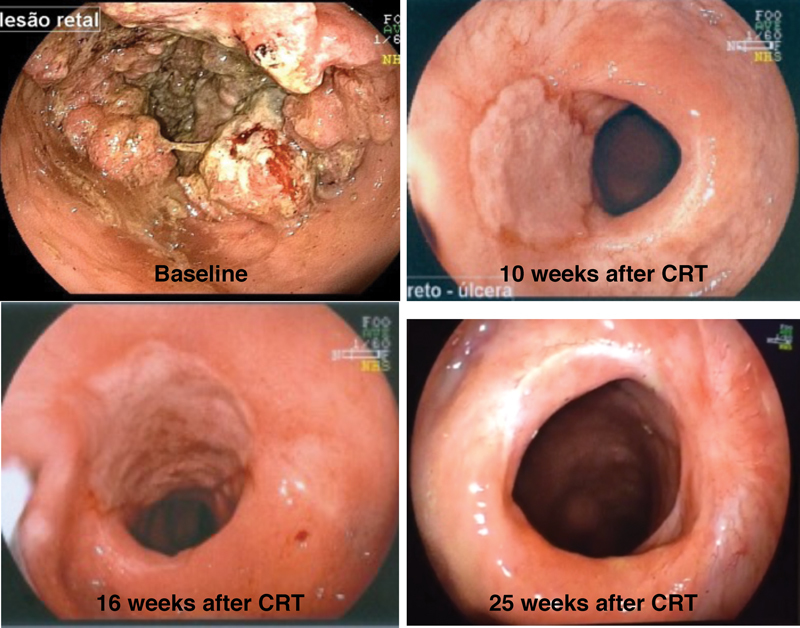
Endoscopic view of the same patient with rectal cancer in different times during treatment. Baseline: rectal tumor before starting chemoradiation therapy (CRT); 10 weeks after CRT: residual ulcer 10 weeks after finishing CRT; 16 weeks after CRT: residual ulcer 16 weeks after finishing CRT; 25 weeks after CRT: complete clinical response with whitening of the mucosa 25 weeks after finishing CRT.
Tools in the Assessment of Tumor Response
Clinical and Endoscopic Assessment
Clinical assessment is one of the most important tools to evaluate tumor response. Commonly, patients with tumor regression would have relief of their symptoms. DRE is an irreplaceable tool for the evaluation of response. The stringent criteria to consider a cCR include the absence of any irregularity, mass, ulceration, or stenosis during the DRE. The surface has to be regular and smooth. 8
Endoscopic evaluation of the area harboring the original tumor is the remaining key component of clinical assessment. It is important to look for any irregularity or superficial ulcers missed during DRE. A flat white scar and telangiectasia are common endoscopic findings among patients with a cCR ( Fig. 2 ). Even though flexible scopes may provide photographic documentation of endoscopic response, rigid proctoscopy may suffice for the majority of patients. 8
Fig. 2.
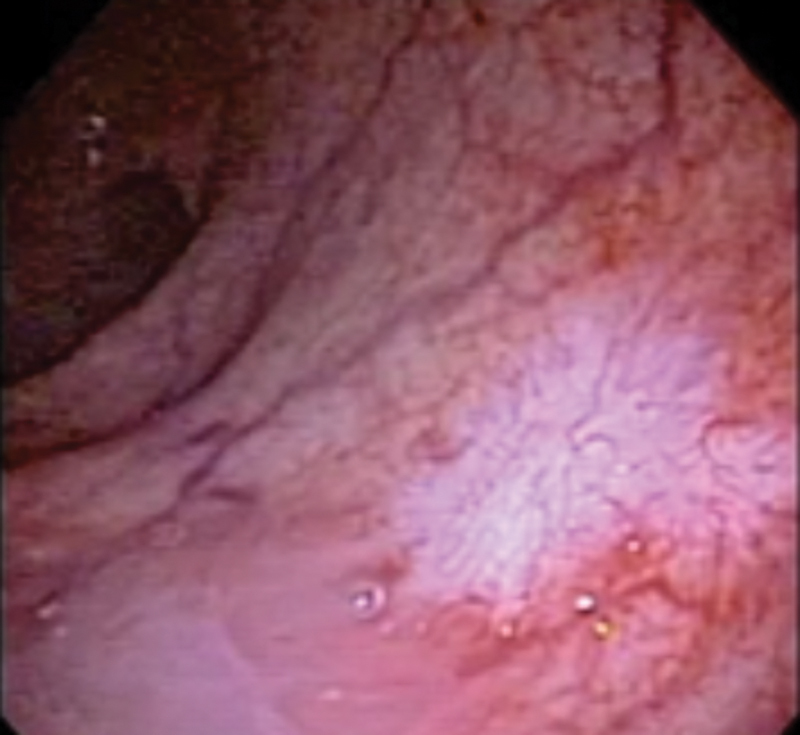
Endoscopic view of complete clinical response after chemoradiation therapy showing whitening of the mucosa and telangiectasia.
In the presence of a cCR by DRE and proctoscopy, endoscopic biopsies are not recommended. Even in the setting of incomplete clinical response (CR), endoscopic biopsy results should be interpreted with caution. Among patients with significant response, negative predictive values of these endoscopic biopsies have been reported to be consistently low. 45 Therefore, a negative biopsy in the setting of incomplete CR does not rule out microscopic residual cancer.
Radiological Assessment
Even though historically the definition of a cCR has been based on clinical and endoscopic findings by direct assessment of rectal wall, radiological studies have always attempted to provide additional information unavailable to the finger or the proctoscope, particularly regarding nodal or mesorectal status of the disease. Currently, however, significant developments in imaging definition and interpretation have resulted in significant increases in accuracy for the assessment of response not only within the mesorectum compartment but also within the rectal wall.
High-resolution magnetic resonance imaging (MRI) is now routinely used for the assessment of response. The ability to discriminate between fibrosis and residual disease has improved with advances in technology, placing the resonance as an essential tool to confirm clinical and endoscopic findings of a cCR. 46 MRI may provide an accurate radiological (magnetic resonance tumor regression grade [mrTRG]) estimate of the pathological TRG. The use of this mrTRG score may identify good and poor responders with significant impact in disease free and overall survival. 47 48
Even though clinical and endoscopic assessment using stringent criteria will result in high specificity rates for the detection of a pCR, a significant amount of patients with incomplete CR will still harbor complete pathological response. 49 50 In fact, it seems that the majority of patients with pCR after nCRT have incomplete CR after 8 to 12 weeks from nCRT. 50 Therefore, there is a potential role for MR studies to identify patients with incomplete CR that may ultimately harbor pCR. Currently, these patients would be referred to immediate radical surgery. However, radiological tools may be able to accurately identify these patients and avoid potentially unnecessary surgery. 51
Recently, a study that compared mrTRG and residual mucosal abnormalities following nCRT suggested that mrTRG system may identify nearly 10 times more complete pathological responses compared with clinical endoscopic findings. These findings may improve the selection of patients with pCR despite initial incomplete CR, and that may be appropriate candidates for deferral of surgery. 51
Diffusion-weighted MRI (DWI-MRI) may add significant functional information to standard MRI. The fact that diffusion properties of water molecules may vary in areas of tissue necrosis, high cellularity (frequently observed within tumor tissues) or fibrosis, may be used to help assess tumor response to nCRT. The absence of restriction to diffusion of water molecules has been associated with the absence of residual cancer (complete response). On the other hand, restriction to diffusion of water molecules (seen as high signal intensity in the area of the previous tumor) may indicate the presence of residual cancer cells (incomplete response). Initial reports with DWI-MRI for the assessment of response to nCRT have shown promising results with high accuracy rates and may constitute an useful tool during assessment of response. 52 53
Positron emission tomography/computed tomography (PET/CT) imaging has been studied for the prediction of response to CRT. The use of molecular imaging may provide additional information to standard structural/anatomical features to help distinguish between fibrosis and residual tumor. The use of fluoro-2-deoxy- d -glucose (FDG) allows for the estimate of tissue metabolism (standard uptake values [SUV]) within areas of interest, and fused images of PET and CT may indicate precise anatomical areas of residual cancer cells, even among mucinous histological subtypes. 54
Most of the available studies have focused on SUV variation for the identification of complete responders to nCRT using variable interval periods and sequential PET/CT imaging. 22 55 56 Accuracies, however, have been insufficient for its routine recommendation into clinical practice. A recently reported study has suggested the role of combination of SUV variation and volumetric reduction in tumors to predict complete response to nCRT. Using individual technical calibration for determining metabolic tumor volumes estimates, variation in total lesion glycolysis (determined by metabolic tumor volume and mean SUV values) was found to be the best predictor of response to nCRT using sequential PET/CT imaging at baseline and 12 weeks from nCRT completion. 57
Complete Response: Watch-and-Wait Strategy
Watch-and-Wait Strategy: Follow-Up
When a nonoperative strategy for cCR in rectal cancer is considered, a relatively intensive follow-up is certainly required ( Table 1 ). Patients should be encouraged to adhere to this strict follow-up program to allow early recognition of any local or systemic recurrence and therefore increasing the chance of a successful salvage treatment. After initial assessment of response confirming a cCR, visits should be scheduled every 1 to 2 months during the first year, every 3 months during the second year, and every 6 months thereafter. DRE, proctoscopy, and carcinoembryonic antigen (CEA) level determination are recommended for all visits. Timing for radiological assessment during follow-up has not yet been standardized. Routine MRI for the assessment of the rectal wall, mesorectum, and pelvic nodes every 6 months for the first 2 years and yearly thereafter has been our practice. 6
Table 1. Follow-up after complete clinical response for patients included in the wait-and-watch protocol.
| First year | DRE, proctoscopy, and CEA every 1 to 2 mo Pelvic magnetic resonance imaging every 6 mo Systemic evaluation every 6 mo |
| Second year | DRE, proctoscopy, and CEA every 3 mo Pelvic magnetic resonance imaging every 6 mo Systemic evaluation every 6 mo |
| Third to fifth year | DRE, proctoscopy, and CEA every 6 mo Pelvic magnetic resonance imaging yearly Systemic evaluation yearly |
| Fifth year onward | DRE, proctoscopy, and CEA yearly Pelvic magnetic resonance imaging yearly Systemic evaluation yearly |
Abbreviations: CEA, carcinoembryonic antigen; DRE, digital rectal examination.
Outcomes
Patients managed nonoperatively under the WW strategy after a cCR following nCRT were originally reported to have similar long-term oncological outcomes to patients with complete pathological response after radical surgery. 9 Additional retrospective studies reported by others have consistently shown similar oncological outcomes between these subgroups of patients. 31 58 59 60 61 62 63 These findings further support the idea that patients with a cCR may be spared from the surgical morbidity and mortality of radical surgery with no oncological compromise. 5 In addition, functional outcomes of patients managed nonoperatively appear to be better than outcomes of not only radical surgery but also of other organ-preserving strategies (transanal local excision). 3 60
Local recurrences after this treatment strategy are still a concern and may develop at any time during follow-up. The majority of local recurrences appear to develop within the first 12 months of follow-up and may represent limitations in the precise identification of microscopic residual disease among “apparent” complete clinical responders. For these reasons, these “early recurrences” developing within the initial 12 months of follow-up have been called “early regrowths” instead. 4 63 64 Still, close and strict follow-up may allow early detection of regrowths, leading to identical oncological outcomes to patients with incomplete CR immediately after 8 to 12 weeks from CRT completion. 65 In addition, local recurrences (late and early regrowths) are usually amenable to salvage therapies, often allowing sphincter preservation and associated with excellent long-term local disease control. 4
Considering that the rate of complete clinical or pathological response was historically <30% of patients across most of the studies, one could assume that this treatment strategy could benefit a rather limited proportion of patients with rectal cancer. However, the observation of increased rates of complete response (clinical or pathological) using regimens with consolidation chemotherapy and with the inclusion of earlier stages of disease (cT2N0 otherwise candidates for ultralow resections or abdominoperineal resections) may result in nearly 50% that ultimately avoid surgical resection. 25 42 This has been further confirmed in a prospective trial including patients with T2 and T3 rectal cancers managed by CRT and an additional endorectal high-dose brachytherapy boost (total 65 Gy) that showed a 58% cCR rate at 2 years of follow-up without surgical resection. 31
Finally, in the era of evidence-based medicine, a randomized prospective trial is still lacking to definitively demonstrate the oncological equivalence of WW and radical surgery in the setting of a cCR following nCRT. 66 Even though such a trial is not likely to be performed, a recent study using a propensity-score matched cohort analysis comparing WW and radical surgery has been designed to demonstrate noninferiority of the WW approach. Curiously, however, the comparison between groups demonstrated a slight superiority of the nonoperative management of these patients in terms of survival and a clear benefit in colostomy-free survival even when accounting for the development of local recurrences. 63
Adjuvant Treatment
The use of adjuvant systemic chemotherapy following a cCR managed nonoperatively is still a matter of controversy. Most studies have not offered adjuvant chemotherapy to these patients, even though several guidelines may recommend the use of adjuvant therapy based on pretreatment staging features rather than on response to nCRT. This means that a baseline cT3N1 would require adjuvant therapy, whereas a baseline cT2N0 would not, even though both patients develop cCR. However logical this may seem, there are insufficient data to support either strategies (based on pre- or posttreatment status).
In a pooled analysis of patients undergoing nCRT followed by radical surgery, patients with pCR showed an 11% distant metastases rate. 67 Curiously, nearly 40% of these patients had received adjuvant 5FU-based chemotherapy. This compares to 14% distant metastases rate among patients undergoing standard CRT with cCR managed nonoperatively without the use of adjuvant chemotherapy. 4
Finally, with the use of consolidation CRT regimens, the dose of adjuvant systemic therapy may ultimately have been shifted to the neoadjuvant period, rendering the discussion of adjuvant chemotherapy meaningless. However, there are still insufficient data to fully support this.
Conclusions
Organ preservation in the management of rectal cancer has become a valid option for select patients after significant response to neoadjuvant CRT. Patients who develop complete tumor regression with no clinical, endoscopic, or radiological evidence of residual cancer may be offered no immediate surgery and enrolled in a strict surveillance program (WW) with excellent functional and acceptable oncological outcomes.
Reference
- 1.Kosinski L, Habr-Gama A, Ludwig K, Perez R. Shifting concepts in rectal cancer management: a review of contemporary primary rectal cancer treatment strategies. CA Cancer J Clin. 2012;62(03):173–202. doi: 10.3322/caac.21138. [DOI] [PubMed] [Google Scholar]
- 2.Sanghera P, Wong D W, McConkey C C, Geh J I, Hartley A. Chemoradiotherapy for rectal cancer: an updated analysis of factors affecting pathological response. Clin Oncol (R Coll Radiol) 2008;20(02):176–183. doi: 10.1016/j.clon.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Habr-Gama A, Lynn P B, Jorge J MN et al. Impact of organ-preserving strategies on anorectal function in patients with distal rectal cancer following neoadjuvant chemoradiation. Dis Colon Rectum. 2016;59(04):264–269. doi: 10.1097/DCR.0000000000000543. [DOI] [PubMed] [Google Scholar]
- 4.Habr-Gama A, Gama-Rodrigues J, São Julião G P et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys. 2014;88(04):822–828. doi: 10.1016/j.ijrobp.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Smith F M, Rao C, Oliva Perez R et al. Avoiding radical surgery improves early survival in elderly patients with rectal cancer, demonstrating complete clinical response after neoadjuvant therapy: results of a decision-analytic model. Dis Colon Rectum. 2015;58(02):159–171. doi: 10.1097/DCR.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 6.Habr-Gama A, São Julião G P, Perez R O. Nonoperative management of rectal cancer: identifying the ideal patients. Hematol Oncol Clin North Am. 2015;29(01):135–151. doi: 10.1016/j.hoc.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Smith F M, Waldron D, Winter D C. Rectum-conserving surgery in the era of chemoradiotherapy. Br J Surg. 2010;97(12):1752–1764. doi: 10.1002/bjs.7251. [DOI] [PubMed] [Google Scholar]
- 8.Habr-Gama A, Perez R O, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53(12):1692–1698. doi: 10.1007/DCR.0b013e3181f42b89. [DOI] [PubMed] [Google Scholar]
- 9.Habr-Gama A, Perez R O, Nadalin Wet al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results Ann Surg 200424004711–717., discussion 717–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopes-Ramos C, Koyama F C, Habr-Gama A et al. Comprehensive evaluation of the effectiveness of gene expression signatures to predict complete response to neoadjuvant chemoradiotherapy and guide surgical intervention in rectal cancer. Cancer Genet. 2015;208(06):319–326. doi: 10.1016/j.cancergen.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Brettingham-Moore K H, Duong C P, Greenawalt D M et al. Pretreatment transcriptional profiling for predicting response to neoadjuvant chemoradiotherapy in rectal adenocarcinoma. Clin Cancer Res. 2011;17(09):3039–3047. doi: 10.1158/1078-0432.CCR-10-2915. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T, Kobunai T, Akiyoshi T, Matsuda K, Ishihara S, Nozawa K. Prediction of response to preoperative chemoradiotherapy in rectal cancer by using reverse transcriptase polymerase chain reaction analysis of four genes. Dis Colon Rectum. 2014;57(01):23–31. doi: 10.1097/01.dcr.0000437688.33795.9d. [DOI] [PubMed] [Google Scholar]
- 13.Rimkus C, Friederichs J, Boulesteix A L et al. Microarray-based prediction of tumor response to neoadjuvant radiochemotherapy of patients with locally advanced rectal cancer. Clin Gastroenterol Hepatol. 2008;6(01):53–61. doi: 10.1016/j.cgh.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Hardiman K M, Ulintz P J, Kuick R D et al. Intra-tumor genetic heterogeneity in rectal cancer. Lab Invest. 2016;96(01):4–15. doi: 10.1038/labinvest.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bettoni F, Masotti C, Habr-Gama A et al. Intratumoral genetic heterogeneity in rectal cancer: are single biopsies representative of the entirety of the tumor? Ann Surg. 2017;265(01):e4–e6. doi: 10.1097/SLA.0000000000001937. [DOI] [PubMed] [Google Scholar]
- 16.Perez R O, Habr-Gama A, São Julião G P et al. Should we give up the search for a clinically useful gene signature for the prediction of response of rectal cancer to neoadjuvant chemoradiation? Dis Colon Rectum. 2016;59(09):895–897. doi: 10.1097/DCR.0000000000000620. [DOI] [PubMed] [Google Scholar]
- 17.Sauer R, Becker H, Hohenberger W et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 18.Kapiteijn E, Marijnen C A, Nagtegaal I D et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(09):638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 19.van Gijn W, Marijnen C A, Nagtegaal I D et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12(06):575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 20.Sauer R, Liersch T, Merkel S et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 21.Peeters K C, van de Velde C J, Leer J W et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J Clin Oncol. 2005;23(25):6199–6206. doi: 10.1200/JCO.2005.14.779. [DOI] [PubMed] [Google Scholar]
- 22.Perez R O, Habr-Gama A, Gama-Rodrigues J et al. Accuracy of positron emission tomography/computed tomography and clinical assessment in the detection of complete rectal tumor regression after neoadjuvant chemoradiation: long-term results of a prospective trial (National Clinical Trial 00254683) Cancer. 2012;118(14):3501–3511. doi: 10.1002/cncr.26644. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Aguilar J, Shi Q, Thomas C R, Jr et al. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol. 2012;19(02):384–391. doi: 10.1245/s10434-011-1933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lezoche E, Baldarelli M, Lezoche G, Paganini A M, Gesuita R, Guerrieri M. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg. 2012;99(09):1211–1218. doi: 10.1002/bjs.8821. [DOI] [PubMed] [Google Scholar]
- 25.Habr-Gama A, Sabbaga J, Gama-Rodrigues J et al. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum. 2013;56(10):1109–1117. doi: 10.1097/DCR.0b013e3182a25c4e. [DOI] [PubMed] [Google Scholar]
- 26.Bosset J F, Calais G, Mineur L et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results--EORTC 22921. J Clin Oncol. 2005;23(24):5620–5627. doi: 10.1200/JCO.2005.02.113. [DOI] [PubMed] [Google Scholar]
- 27.Radu C, Berglund A, Påhlman L, Glimelius B. Short-course preoperative radiotherapy with delayed surgery in rectal cancer - a retrospective study. Radiother Oncol. 2008;87(03):343–349. doi: 10.1016/j.radonc.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 28.Wiltshire K L, Ward I G, Swallow C et al. Preoperative radiation with concurrent chemotherapy for resectable rectal cancer: effect of dose escalation on pathologic complete response, local recurrence-free survival, disease-free survival, and overall survival. Int J Radiat Oncol Biol Phys. 2006;64(03):709–716. doi: 10.1016/j.ijrobp.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Jakobsen A, Ploen J, Vuong T, Appelt A, Lindebjerg J, Rafaelsen S R. Dose-effect relationship in chemoradiotherapy for locally advanced rectal cancer: a randomized trial comparing two radiation doses. Int J Radiat Oncol Biol Phys. 2012;84(04):949–954. doi: 10.1016/j.ijrobp.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Gerard J P, Frin A C, Doyen J et al. Organ preservation in rectal adenocarcinoma (T1) T2-T3 Nx M0. Historical overview of the Lyon Sud - Nice experience using contact x-ray brachytherapy and external beam radiotherapy for 120 patients. Acta Oncol. 2015;54(04):545–551. doi: 10.3109/0284186X.2014.975840. [DOI] [PubMed] [Google Scholar]
- 31.Appelt A L, Pløen J, Harling H et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16(08):919–927. doi: 10.1016/S1470-2045(15)00120-5. [DOI] [PubMed] [Google Scholar]
- 32.Vuong T, Devic S, Podgorsak E. High dose rate endorectal brachytherapy as a neoadjuvant treatment for patients with resectable rectal cancer. Clin Oncol (R Coll Radiol) 2007;19(09):701–705. doi: 10.1016/j.clon.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Schrag D, Weiser M R, Goodman K Aet al. Neoadjuvant FOLFOX-bev, without radiation, for locally advanced rectal cancerJ Clin Oncol 2010;28(suppl):abstr 3511
- 34.Habr-Gama A, Fernandez L M, Perez R O. What more do we want from neoadjuvant treatment strategies in rectal cancer? Colorectal Cancer. 2015;4:1–4. [Google Scholar]
- 35.Habr-Gama A, Perez R O, Sabbaga J, Nadalin W, São Julião G P, Gama-Rodrigues J. Increasing the rates of complete response to neoadjuvant chemoradiotherapy for distal rectal cancer: results of a prospective study using additional chemotherapy during the resting period. Dis Colon Rectum. 2009;52(12):1927–1934. doi: 10.1007/DCR.0b013e3181ba14ed. [DOI] [PubMed] [Google Scholar]
- 36.Brown G.Thin section MRI in multidisciplinary pre-operative decision making for patients with rectal cancer Br J Radiol 200578(Spec No 2):S117–S127. [DOI] [PubMed] [Google Scholar]
- 37.Francois Y, Nemoz C J, Baulieux J et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17(08):2396. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 38.Tulchinsky H, Shmueli E, Figer A, Klausner J M, Rabau M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15(10):2661–2667. doi: 10.1245/s10434-008-9892-3. [DOI] [PubMed] [Google Scholar]
- 39.Kalady M F, de Campos-Lobato L F, Stocchi L et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250(04):582–589. doi: 10.1097/SLA.0b013e3181b91e63. [DOI] [PubMed] [Google Scholar]
- 40.Evans J, Tait D, Swift I et al. Timing of surgery following preoperative therapy in rectal cancer: the need for a prospective randomized trial? Dis Colon Rectum. 2011;54(10):1251–1259. doi: 10.1097/DCR.0b013e3182281f4b. [DOI] [PubMed] [Google Scholar]
- 41.Wolthuis A M, Penninckx F, Haustermans K et al. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol. 2012;19(09):2833–2841. doi: 10.1245/s10434-012-2327-1. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Aguilar J, Chow O S, Smith D D et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(08):957–966. doi: 10.1016/S1470-2045(15)00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lefevre J H, Mineur L, Kotti Set al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6) J Clin Oncol 2016(e-pub ahead of print).JCO676049. [DOI] [PubMed] [Google Scholar]
- 44.Perez R O, Habr-Gama A, São Julião G P et al. Optimal timing for assessment of tumor response to neoadjuvant chemoradiation in patients with rectal cancer: do all patients benefit from waiting longer than 6 weeks? Int J Radiat Oncol Biol Phys. 2012;84(05):1159–1165. doi: 10.1016/j.ijrobp.2012.01.096. [DOI] [PubMed] [Google Scholar]
- 45.Perez R O, Habr-Gama A, Pereira G V et al. Role of biopsies in patients with residual rectal cancer following neoadjuvant chemoradiation after downsizing: can they rule out persisting cancer? Colorectal Dis. 2012;14(06):714–720. doi: 10.1111/j.1463-1318.2011.02761.x. [DOI] [PubMed] [Google Scholar]
- 46.Lambregts D M, Maas M, Bakers F C et al. Long-term follow-up features on rectal MRI during a wait-and-see approach after a clinical complete response in patients with rectal cancer treated with chemoradiotherapy. Dis Colon Rectum. 2011;54(12):1521–1528. doi: 10.1097/DCR.0b013e318232da89. [DOI] [PubMed] [Google Scholar]
- 47.Patel U B, Taylor F, Blomqvist L et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29(28):3753–3760. doi: 10.1200/JCO.2011.34.9068. [DOI] [PubMed] [Google Scholar]
- 48.Patel U B, Brown G, Rutten H et al. Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann Surg Oncol. 2012;19(09):2842–2852. doi: 10.1245/s10434-012-2309-3. [DOI] [PubMed] [Google Scholar]
- 49.Nahas S C, Rizkallah Nahas C S, Sparapan Marques C F et al. Pathologic complete response in rectal cancer: can we detect it? Lessons learned from a proposed randomized trial of watch-and-wait treatment of rectal cancer. Dis Colon Rectum. 2016;59(04):255–263. doi: 10.1097/DCR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 50.Smith F M, Wiland H, Mace A, Pai R K, Kalady M F. Clinical criteria underestimate complete pathological response in rectal cancer treated with neoadjuvant chemoradiotherapy. Dis Colon Rectum. 2014;57(03):311–315. doi: 10.1097/DCR.0b013e3182a84eba. [DOI] [PubMed] [Google Scholar]
- 51.Bhoday J, Smith F, Siddiqui M R et al. Magnetic resonance tumor regression grade and residual mucosal abnormality as predictors for pathological complete response in rectal cancer postneoadjuvant chemoradiotherapy. Dis Colon Rectum. 2016;59(10):925–933. doi: 10.1097/DCR.0000000000000667. [DOI] [PubMed] [Google Scholar]
- 52.Lambregts D M, Vandecaveye V, Barbaro B et al. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol. 2011;18(08):2224–2231. doi: 10.1245/s10434-011-1607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Curvo-Semedo L, Lambregts D M, Maas M et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy--conventional MR volumetry versus diffusion-weighted MR imaging. Radiology. 2011;260(03):734–743. doi: 10.1148/radiol.11102467. [DOI] [PubMed] [Google Scholar]
- 54.Dos Anjos D A, Habr-Gama A, Vailati B B et al. (18)F-FDG uptake by rectal cancer is similar in mucinous and nonmucinous histological subtypes. Ann Nucl Med. 2016;30(08):513–517. doi: 10.1007/s12149-016-1089-4. [DOI] [PubMed] [Google Scholar]
- 55.Cascini G L, Avallone A, Delrio P et al. 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. J Nucl Med. 2006;47(08):1241–1248. [PubMed] [Google Scholar]
- 56.Kristiansen C, Loft A, Berthelsen A K et al. PET/CT and histopathologic response to preoperative chemoradiation therapy in locally advanced rectal cancer. Dis Colon Rectum. 2008;51(01):21–25. doi: 10.1007/s10350-007-9095-1. [DOI] [PubMed] [Google Scholar]
- 57.Dos Anjos D A, Perez R O, Habr-Gama A et al. Semiquantitative volumetry by sequential PET/CT may improve prediction of complete response to neoadjuvant chemoradiation in patients with distal rectal cancer. Dis Colon Rectum. 2016;59(09):805–812. doi: 10.1097/DCR.0000000000000655. [DOI] [PubMed] [Google Scholar]
- 58.Vaccaro C A, Yazyi F J, Ojra Quintana G et al. Locally advanced rectal cancer: preliminary results of rectal preservation after neoadjuvant chemoradiotherapy [in Spanish] Cir Esp. 2016;94(05):274–279. doi: 10.1016/j.ciresp.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 59.Araujo R O, Valadão M, Borges D et al. Nonoperative management of rectal cancer after chemoradiation opposed to resection after complete clinical response. A comparative study. Eur J Surg Oncol. 2015;41(11):1456–1463. doi: 10.1016/j.ejso.2015.08.156. [DOI] [PubMed] [Google Scholar]
- 60.Maas M, Beets-Tan R G, Lambregts D M et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29(35):4633–4640. doi: 10.1200/JCO.2011.37.7176. [DOI] [PubMed] [Google Scholar]
- 61.Dalton R S, Velineni R, Osborne M E et al. A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Colorectal Dis. 2012;14(05):567–571. doi: 10.1111/j.1463-1318.2011.02752.x. [DOI] [PubMed] [Google Scholar]
- 62.Smith R K, Fry R D, Mahmoud N N, Paulson E C. Surveillance after neoadjuvant therapy in advanced rectal cancer with complete clinical response can have comparable outcomes to total mesorectal excision. Int J Colorectal Dis. 2015;30(06):769–774. doi: 10.1007/s00384-015-2165-2. [DOI] [PubMed] [Google Scholar]
- 63.Renehan A G, Malcomson L, Emsley R et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17(02):174–183. doi: 10.1016/S1470-2045(15)00467-2. [DOI] [PubMed] [Google Scholar]
- 64.Martens M H, Maas M, Heijnen L A et al. Long-term outcome of an organ preservation program after neoadjuvant treatment for rectal cancer. J Natl Cancer Inst. 2016;108(12):108. doi: 10.1093/jnci/djw171. [DOI] [PubMed] [Google Scholar]
- 65.Habr-Gama A, Perez R O, Proscurshim I et al. Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: does delayed surgery have an impact on outcome? Int J Radiat Oncol Biol Phys. 2008;71(04):1181–1188. doi: 10.1016/j.ijrobp.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 66.Perez R O. Complete clinical response in rectal cancer: a turning tide. Lancet Oncol. 2016;17(02):125–126. doi: 10.1016/S1470-2045(15)00487-8. [DOI] [PubMed] [Google Scholar]
- 67.Maas M, Nelemans P J, Valentini V et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(09):835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]


