Summary
Peripheral nociceptive stimuli from orofacial structures are largely transmitted by the trigeminal nerve. According to the peripheral noxious stimuli, neurons in the trigeminal ganglion (TG) produce neuropeptides such as substance P, and calcitonin-gene-related peptide, etc. Beside the production of neuropeptides, there exists unique non-synaptic interaction system between maxillary and mandibular neurons in the TG. Neurons in the TG are surrounded by satellite glial cells (SGCs), which initially receive the signal from TG neurons. These activated SGCs secrete a transmitter to activate adjacent SGCs or TG neurons, thereby amplifying the signal, for example, from mandibular neurons to maxillary neurons in the TG. Similar to the dorsal root ganglion, in the TG, microglia/macrophage-like cells (MLCs) are activated by uptake of a transmitter from TG neurons or SGCs. This communication between neurons, SGCs, and MLCs results in responses such as ectopic pain, hyperesthesia, or allodynia. The focus of this review is the cooperative interaction of the maxillary and mandibular nerves in the TG by neuropeptides, and adenosine 3′-phosphate (ATP) signaling from neurons to SGCs and MLCs. Stimulated neurons either secrete ATP by means of vesicular nucleotide transporters, or secrete neuropeptides from the neuronal cell body to mediate signal transmission.
Abbreviations: TG, trigeminal ganglion; DRG, dorsal root ganglion; SGC, satellite glial cell; MLC, microglia/macrophage-like cell; ATP, adenosine 3′-phosphate; VNUT, vesicular nucleotide transporter; SP, substance P; CGRP, calcitonin-gene-related peptide; VIP, vasoactive intestinal peptide; PACAP, pituitary adenylate-cyclase-activating polypeptide receptor type 1
Keywords: Trigeminal ganglion, Neuropeptides, ATP, Neuron, Satellite glial cell
1. Introduction
Peripheral nociceptive stimuli from intra- and extra-oral structures are largely transmitted by the trigeminal nerve. The primary afferent neurons of the trigeminal nerve are mainly located in the trigeminal ganglion (TG) and partially localized in the mesencephalic trigeminal nucleus in the brain stem. The trigeminal nerve consists of three main branches: the ophthalmic (V1), maxillary (V2), and mandibular (V3) nerves, each of which provides somatosensory innervation of a specific region of the head. Trigeminal nerve neurons are pseudounipolar and are distributed within distinct areas of the TG. In the rat, the neuronal distribution is evident in the rostral and caudal regions of the TG [1].
Neuropathic pain caused by peripheral nerve injury is common following procedures such as tooth extraction and often leads to ectopic orofacial pain [2] via two possible mechanisms. The first is related to the coincidental cross-migration of V2 and V3 neurons such that they are found outside their respective regions. In fact, some V2 neurons are located in the region of V3 neurons and vice versa. Alternatively, neurotransmitters secreted by damaged neuron affect not only adjacent neurons but also neurons in other regions.
The signal from TG neurons is initially transmitted to the surrounding satellite glial cells (SGCs) via the gap junctions between them [3]. Neurotransmitters secreted in TG neurons may also act on SGCs through the latter’s surface receptors. SGCs activated by TG neurons secrete transmitters, which act on adjacent SGCs or TG neurons and result in signal amplification, including from the region of V3 neurons to that of V2 neurons. In addition, as in the dorsal root ganglion (DRG), responses such as hyperesthesia or allodynia are enhanced by microglia distributed in the TG that are activated by neurotransmitters released by TG neurons or SGCs.
The focus of this review is the molecular signaling from TG neurons to SGCs and from SGCs to other cells, including other TG neurons, SGCs, and microglia/macrophage-like cells (MLCs). We also discuss the signaling molecules used by these cells, particularly neuropeptides and adenosine 3′-phosphate (ATP), and the possible role of the non-synaptic interaction system between maxillary and mandibular neurons in TG.
2. Neuropeptides in TG neurons
Neurons of the TG secrete several different neuropeptides, including substance P (SP), calcitonin gene-related peptide (CGRP), vasoactive intestinal peptide (VIP), pituitary adenylate-cyclase-activating polypeptide receptor type 1 (PACAP), neuropeptide Y (NPY), and somatostatin (SS). These neuropeptides are present in neurons, and by acting as neuromediators or neuromodulators, are involved in signal transmission. The neuropeptides in the TG were previously well reviewed by Lazarov [4]; here we present several recent findings on this group of signaling molecules.
2.1. Substance P
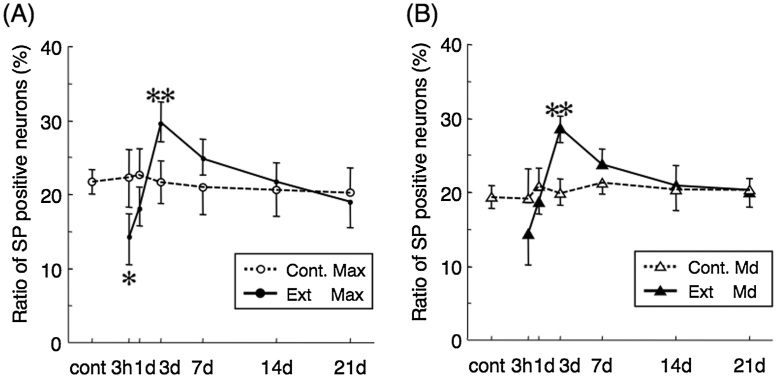
As a member of the tachykinin family of neuropeptides, SP is distributed not only in neurons but also in the cells of peripheral tissues [5], [6]. Accordingly, its effects are not limited to the nervous system but are more extensive. In the TG, >99% of the nerve fibers that store SP are unmyelinated, and most are small and medium-sized neurons (cross-sectional area <800 m2) [7]. In untreated animals, SP-containing cells with diameters of 15–50 μm are distributed throughout the TG and comprise 10–30% of all TG cells [8]. SP-containing afferents are unmyelinated C-fibers in which SP is secreted from both central and peripheral nerve terminals [9]. Although SP can be released from terminals within the brain stem and peripheral tissues, its transport is mostly to the latter [10]. SP in TG neurons may be associated with neurogenic inflammation, a tissue reaction that develops in acute conditions such as wound repair, in which damaged TG neurons release SP, resulting in a temporal decrease in SP-immunoreactive (IR) neurons. The injury of peripheral neurons induces an increase in SP production; however, the increase in the proportion of SP-IR neurons occurs not only in the maxillary nerve but also in mandibular nerves (Fig. 1). Because primary afferent neurons in TG are anatomically isolated from one another and not synaptically interconnected, other means of interaction must exist between maxillary and mandibular nerves. Similarly, neurokinin 1-receptor (NK1-R), which has a high affinity for SP, is also found in TG neurons and its expression in both maxillary and mandibular nerve regions increases after injury induced by maxillary molar extraction [11]. These findings suggest that peripheral nerve injury upregulates neuropeptide expression to cause an autocrine-like reaction in the TG. Additional studies are needed to better understand the synergistic upregulation of SP and its receptor in maxillary and mandibular regions of the TG in response to injury.
Figure 1.
The proportion of SP-immunoreactive (IR) neurons per PGP-9.5-IR neuron (all neuron) in maxillary (A) and mandibular nerve region (B) in the TG at 3 h to 21 days after the maxillary first molar extraction. Control proportion indicated as Cont. Max (maxillary nerve region) and Cont. Md (mandibular nerve region) and extracted proportion indicated as Ext Max and Ext Md. Mean ± SD. Significant differences from control value at each time point (*p < 0.05 and **p < 0.01, tested by one-way ANOVA).
2.2. Calcitonin-gene-related peptide
CGRP is widely expressed within the trigeminal pain pathway, both in the peripheral nervous system (PNS) and the central nervous system (CNS). CGRP has two isoforms, α- and β-CGRP. Where α-CGRP is widely distributed in both the PNS and CNS [12], β-CGRP is found in the enteric nervous system and pituitary gland [13]. α-CGRP is predominantly expressed in TG neurons and is a key neuropeptide involved in both neural and vascular responses. CGRP is present in small neuronal cells and ∼50% of TG neurons are CGRP-positive, whereas SP is present in ∼33% [14]. However, all SP-containing TG cells are also immunopositive for CGRP [15], [16]. Studies in guinea pigs have shown that most neurons expressing SP are nociceptors, whereas those expressing CGRP are either nociceptive or non-nociceptive [17]. SP- and CGRP-containing sensory nerve terminals have been identified in the gingivae and periodontium of many species, including rats and humans [18], [19], [20]. The expression of CGRP is increased in local nerve terminals innervating the inflamed periodontium [21], and the release of this neuropeptide from primary afferents modulates pain transmission through interactions with SP and excitatory amino acids [22], [23]. The role of this neuropeptide in trigeminal vascular nociception was confirmed by the discovery of an association between the sensitization of nociceptive systems and CGRP release from the TG [24], [25]. Moreover, there is evidence supporting the involvement of CGRP in migraine, as its levels in the serum, cerebrospinal fluid, and saliva of migraine patients are increased during migraine attacks [26], [27], [28], [29]. In addition, the administration of triptans that are serotonin receptor agonists, and CGRP inhibitors leads to a reduction in circulating CGRP levels [30], and the intravenous infusion of small doses of CGRP induces migraine in patients suffering from migraines characterized by aura formation [31]. In TG neurons, prolonged treatment with acetaminophen that is the inhibitor of cyclooxygenase, induces an increase in CGRP expression [32]. These findings suggest that several agents, including both small-molecule CGRP receptor antagonists and monoclonal antibodies, would be effective for the treatment of migraines [33].
2.3. VIP and PACAP
VIP and PACAP belong to the secretin/glucagon/VIP superfamily of neuropeptides. VIP was first isolated as a very potent neuropeptide from ovine intestine [34] and was subsequently found to be widely distributed in the CNS and PNS [35], [36]. The biological effects of VIP in mammals include embryonic brain development, pain perception, and inflammation [37]. VIP-positive cells constitute 10–12% of the total TG neurons [38], [39] and are located in the vicinity of SP-containing TG cells. PACAP was originally isolated from rat hypothalamus [40] and occurs in two forms: C-terminally truncated PACAP-27 and PACAP-38 (27 or 38 amino acids). PACAP shares two-thirds sequence homology with the N-terminal domain of VIP. Both forms of PACAP have a broad range of biological effects including vasodilation, relaxation of the lower airways, immune modulation, the stimulation of cell proliferation and differentiation, control of neurotransmitter release, and pain transmission [37], [41]. PACAP is found in a subpopulation of small- to medium-size TG neurons and its actions are mediated by members of the family of seven-transmembrane G protein-coupled receptors [41], [42], [43].
2.4. Neuropeptide Y
Only a few TG neurons are positive for neuropeptide Y (NPY), which is normally produced in sympathetic neurons [44]. Thus, the role of NPY in TG neurons was assumed to be quite limited, but after transection of the mental nerve the expression of NPY in TG neurons increases [45]. More recently, Magnussen et al. [46] found that after peripheral injury, newly synthesized NPY in the TG is transported axonally to the ends of sensory fibers close to the site of tissue injury. However, most of the NPY secreted by sympathetic fibers at the injury site is not taken up by sensory axons. Thus, the role of NPY produced in TG neurons after peripheral injury remains to be determined.
2.5. Somatostatin
The neurotransmitter somatostatin (SS) is synthesized in primary sensory neurons that are mainly found in small ganglion cells [47]. The isoform of SS receptor, SS2A, is also expressed in TG neurons [48]. Although both SS and SP have been found in TG neurons [49], there are fewer SS- than SP-containing neurons [39]. SS inhibits the excitability of rat small trigeminal neurons [50], but enhances the activity of tooth-pulp-evoked cervical horn neurons [51]. The physiological basis of the dual effects of SS on trigeminal nociceptive transmission is still unknown.
2.6. Other peptides
The number of peptide transmitters identified in the TG has progressively increased during the last decade to include galanin [52], orexin [53], nociceptin [54], [55], neurokinin B [56], and hemokinin [57]. Although they were initially thought to be associated with neurogenic inflammation, recent evidence suggests that all of them are also involved in migraine development, plasma protein extravasation, vasodilation of the intracranial vasculature, and peripheral and central sensitization in the trigeminal system [58]. In addition, some trigeminal neuropeptides such as SP, CGRP, neurokinin B, or hemokinin have been found to be associated with bone metabolism at the site of noxious stimulus [59].
3. ATP signaling in the TG
Neuropeptides are involved in the reciprocal activation of TG neurons, but the mechanism of neuron-glia signaling is not well understood. Because there are no intermediate neurons between the maxillary and mandibular neurons, they presumably interact with one or more signaling molecules. Despite the role of neurotrophic factors such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) in signaling between these two cell types, recent attention has focused on ATP, whose many cellular roles include being a neurotransmitter secreted at sensory nerve endings [60], making it a strong candidate signaling molecule. In 1979 ATP was found at vesicle fraction in splenic nerve [61], [62]. Recently, Sawada et al. [63] identified a vesicular nucleotide transporter (VNUT) that delivers ATP to vesicles, allowing its secretion as an extracellular signaling molecule. In a preliminary experiment using an antibody against VNUT, we determined the distribution of VNUT immune-positive vesicles in neurons and satellite glia cells of the TG (Fig. 2). ATP is also a route for cross-talk between glia and neurons. It is stored in and released from many types of neurons, not only in synapses but also in axons and cell bodies [64].
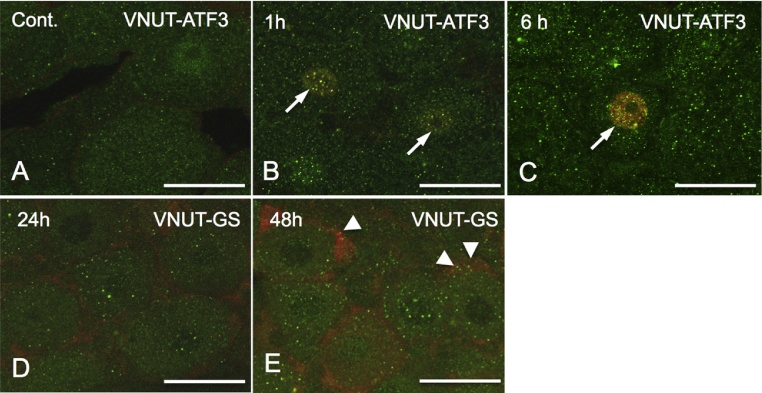
Figure 2.
VNUT distribution in neurons and SGCs in the TG after rat molar extraction. (A–C)Double immunofluorescence staining using antibodies against VNUT (ATP transporter) and Activating Transcription Factor 3 (ATF3: a marker damaged neuron). VNUT-immunoreactive (IR) vesicles localize even in non-damaged TG neuron (A). (B, C) After 1 and 6 h after upper first molar extraction, the nuclei of damaged neurons were immunopositive for ATF3 (arrows) and the number of VNUT-IR vesicles in the cytoplasm of ATF3-immunopositive neurons increased in time-dependent manner. (D, E) Double immunofluorescence staining using antibodies against VNUT (ATP transporter) and glucose synthase (GS: a marker of SGCs). At 24 h after tooth extraction, few VNUT-IR vesicles exhibited in GS-immunopositive SGCs, however, at 48 h after extraction some VNUT-IR vesicles (arrowheads) distributed in SGCs. Bars = 10 μm.
3.1. ATP and ATP receptors in the TG
As an extracellular neurostimulator, ATP activates the metabotropic P2Y and ionotropic P2X receptor families [65]. P2X receptors (P2X R) on sensory neurons are involved in pain transmission [66]. Among the seven subtypes of P2X receptors (P2X1–7), P2X3R mediates nociceptive signals and is expressed on a subset of predominantly small (presumably nociceptive) sensory neurons, including their central terminals [67]. P2X3R is also expressed on TG neurons, which predominantly localize at small- to medium-sized cells and at some large neuronal somata [68], [69]. In addition to P2X3R, SGCs also express P2Y12R [70]. The activation of P2Y12R on SGCs of the TG following lingual nerve injury plays a role in the enhancement of TG neuronal activity and nociceptive reflex behavior, resulting in neuropathic pain in the tongue.
3.2. Neuron-glia signaling in the TG
Neurons in the DRG use ATP to also communicate with SGCs, the main type of glial cells surrounding neuronal cell bodies in ganglia. Electrical stimulation of DRG neurons elicits vesicular ATP release from neuronal cell bodies [64]. Neuronally derived ATP activates P2X7R on adjacent SGCs; conversely, signal return to neurons is mediated by the release of the inflammatory cytokines IL-1β and TNFα, or by neurotorophic factors such as NGF and BDNF. By releasing cytokines, SGCs strengthen neuronal excitability, leading to an auto-amplifying loop of excitation [71], [72]. Shinoda and Iwata [73] introduced a model of ectopic orofacial pain in the rat whisker pad, in which NGF and CGRP induced following local inflammation in the lower lip increase TRPV1 and P2X3R expression in TG neurons. In addition to neurons and SGCs, immune-like glial cells, such as Schwann cells, microglia, and macrophages, responsive to inflammatory cytokines are thought to be involved in ATP signaling in the DRG or TG [74], [75]. In Schwann cells, ATP is a potent activation-dependent signaling molecule between neurons and Schwann cells at both synaptic and non-synaptic regions [76], [77]. Activity-dependent interactions between axons and myelinating glia occur following the non-synaptic release of ATP from axons. Stimulation of P2X R and P2Y R receptors by neuronally released ATP inhibits Schwann cell proliferation, differentiation, and myelination [77], [78], [79]. Macrophages and microglia are additional, newly identified targets of ATP mediated neuron-glia signaling. Whether Iba-1 positive cells in the TG are macrophage-like or microglia-like cells, MLCs also possess ATP receptors. In several models of pain induced by traumatic peripheral lesions, an increase in microglia number without a specific pattern of distribution was observed [80], [81]; in some studies, macrophages in the DRG were activated after nerve injury [75], [82]. It is interesting to note that the application of ATP alone promotes microglial motility, while several neurotransmitters (e.g., glutamate, SP, GABA, serotonin), neurotrophic factors (NGF, BDNF) and chemokines (e.g., CCL2, CX3CL1), and even direct nerve stimulation have no effect [83], [84]. ATP, derived from multiple sources including nerve terminals, induces BDNF release from microglia by activating the P2X4R [85]. Microglial BDNF binds to the TrkB receptor on neurons to induce a shift in the chloride anion gradient in TG neurons. Schematic diagram of ATP mediated neuron-SGCs-MLCs signaling in the TG is indicated in Fig. 3.
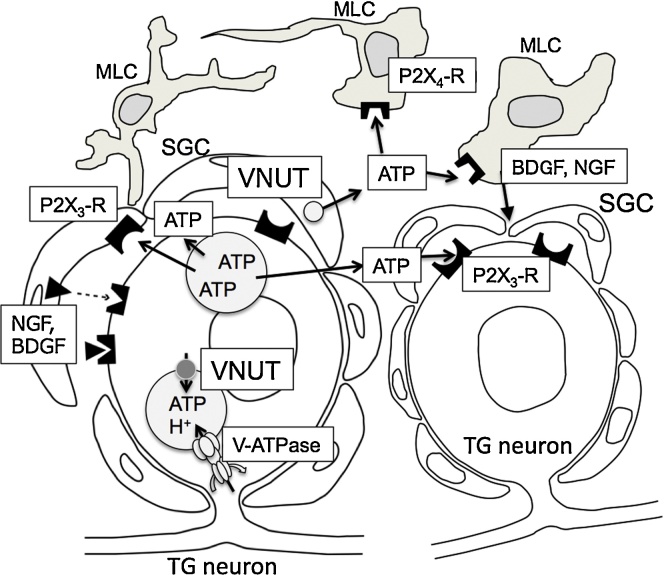
Figure 3.
Schematic diagram of ATP mediated neuron-SGCs-MLCs signaling in the TG. Once nociceptive signal reach TG neuron, the formation of the ATP containing vesicle increases by the cooperation of VNUT and V-ATPase. After the activation of TG neuron, the signal then reaches SGCs, and SGCs also produce ATP containing vesicles by the action of VNUT. Vesicles containing ATP in TG neurons and SGCs moved periphery and release ATP to extracellular space. Extracellular ATP binds ATP receptor (P2X3, P2X4, etc.) on adjacent SGCs, MLCs, or TG neurons. Released ATP would activate SGCs or MLCs and induce to secrete neurotrophic factors such as BDGF or NGF.
4. Concluding remarks
In this review we presented the general aspects of neuropeptide and ATP signaling in the TG. Although there have been many studies aimed at elucidating the neural pathway of orofacial pain, their main focus has been the trigeminal nucleus, thalamus, and primary somatosensory area, not the TG. Nerve cells in the TG do not simply receive a signal from the periphery but modify that signal in a coordinated manner with neighboring neurons, SGCs, and MLCs. This review addressed both the cooperative interactions of the maxillary and mandibular nerves in the TG and neuronal signaling via ATP to SGCs and MLCs (Fig. 4). Stimulated neurons either secrete ATP through vesicles in association with VNUT, or secrete neuropeptides from the neuronal cell body; both result in signal transmission. Each neuropeptide has a specific role in the TG, but how neurons manage their array of neuropeptides must still be elucidated. Similar to the DRG, neuron-SGC-MLC communication is also a feature of the TG and this unique non-synaptic interaction system between maxillary and mandibular neurons in the TG would be associated with hyperesthesia or allodynia. Additional studies are needed to reveal the role of TG in the cellular modification related to signal transduction resulting in orofacial pain.
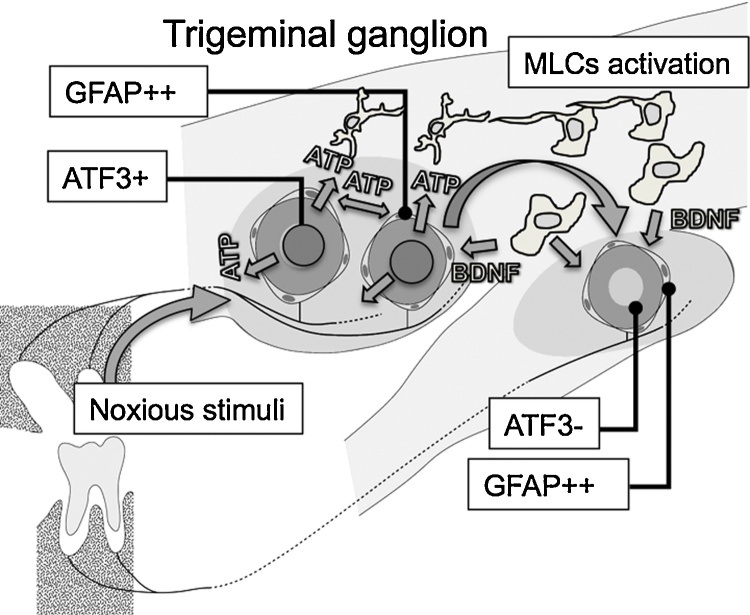
Figure 4.
Schematic diagram of the cooperative interactions of the maxillary and mandibular nerves in the TG, accompanied with the neuronal signalling via ATP to SGCs and MLCs. Stimulated maxillary neurons shows positive for ATF3 (GFAP: glial fibrillary acidic protein, a marker of activated glial cells) and the nearby SGCs become GFAP positive (a maker of activated glial cell). Damaged maxillary neurons secrete ATP or neuropeptides from the neuronal cell body. Secreted ATP or unknown factors would induce the activation of MLCs, and the activated MLCs would secrete some neurotrophic factors such as brain-derived neurotrophic factor (BDNF). BDNF stimulates ATF3 negative maxillary and mandibular neurons surrounded by GFAP positive SGCs.
Conflicts of interest
The authors have no competing financial interests to declare.
Acknowledgment
This work was supported by a JSPS KAKENHI grant (number 26462791) to TG.
References
- 1.Dixon A.D. The ultrastructure of nerve fibers in the trigeminal ganglion of the rat. J Ultrastruct Res. 1963;8:107–121. doi: 10.1016/s0022-5320(63)80023-4. [DOI] [PubMed] [Google Scholar]
- 2.Kaji K., Shinoda M., Honda K., Unno S., Shimizu N., Iwata K. Connexin 43 contributes to ectopic orofacial pain following inferior alveolar nerve injury. Mol Pain. 2016;12 doi: 10.1177/1744806916633704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pannese E., Ledda M., Cherkas P.S., Huang T.Y., Hanani M. Satellite cell reactions to axon injury of sensory ganglion neurons: increase in number of gap junctions and formation of bridges connecting previously separate perineuronal sheaths. Anat Embryol (Berl) 2003;206(5):337–347. doi: 10.1007/s00429-002-0301-6. [DOI] [PubMed] [Google Scholar]
- 4.Lazarov N.E. Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog Neurobiol. 2002;66(1):19–59. doi: 10.1016/s0301-0082(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 5.Maggi C.A., Patacchini R., Rovero P., Giachetti A. Tachykinin receptors and tachykinin receptor antagonists. J Auton Pharmacol. 1993;13(1):23–93. doi: 10.1111/j.1474-8673.1993.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 6.Otsuka M., Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol Rev. 1993;73(2):229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- 7.Bae J.Y., Kim J.H., Cho Y.S., Mah W., Bae Y.C. Quantitative analysis of afferents expressing substance P: calcitonin gene-related peptide, isolectin B4, neurofilament 200, and Peripherin in the sensory root of the rat trigeminal ganglion. J Comp Neurol. 2015;523(1):126–138. doi: 10.1002/cne.23672. [DOI] [PubMed] [Google Scholar]
- 8.Lehtosalo J.I., Uusitalo H., Stjernschantz J., Palkama A. Substance P-like immunoreactivity in the trigeminal ganglion. A fluorescence, light and electron microscope study. Histochemistry. 1984;80(5):421–427. doi: 10.1007/BF00495429. [DOI] [PubMed] [Google Scholar]
- 9.Cuello A.C., Del Fiacco M., Paxinos G. The central and peripheral ends of the substance P-containing sensory neurones in the rat trigeminal system. Brain Res. 1978;152(3):499–500. doi: 10.1016/0006-8993(78)91105-8. [DOI] [PubMed] [Google Scholar]
- 10.Brimijoin S., Lundberg J.M., Brodin E., Hokfelt T., Nilsson G. Axonal transport of substance P in the vagus and sciatic nerves of the guinea pig. Brain Res. 1980;191:443–457. doi: 10.1016/0006-8993(80)91293-7. [DOI] [PubMed] [Google Scholar]
- 11.Gunjigake K.K., Goto T., Nakao K., Konoo T., Kobayashi S., Yamaguchi K. Correlation between the appearance of neuropeptides in the rat trigeminal ganglion and reinnervation of the healing root socket after tooth extraction. Acta Histochem Cytochem. 2006;39(3):69–77. doi: 10.1267/ahc.05057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenfeld M.G., Mermod J.J., Amara S.G., Swanson L.W., Sawchenko P.E., Rivier J. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983;304(5922):129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- 13.Mulderry P.K., Ghatei M.A., Spokes R.A., Jones P.M., Pierson A.M., Hamid Q.A. Differential expression of alpha-CGRP and beta-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience. 1988;25(1):195–205. doi: 10.1016/0306-4522(88)90018-8. [DOI] [PubMed] [Google Scholar]
- 14.Reuss S., Riemann R., Vollrath L. Substance P- and calcitonin gene-related peptide-like immunoreactive neurons in the rat trigeminal ganglion-with special reference to meningeal and pineal innervation. Acta Histochem. 1992;92(1):104–109. doi: 10.1016/S0065-1281(11)80146-7. [DOI] [PubMed] [Google Scholar]
- 15.Skofitsch G., Jacobowitz D.M. Calcitonin gene-related peptide coexists with substance P in capsaicin sensitive neurons and sensory ganglia of the rat. Peptides. 1985;6(4):747–754. doi: 10.1016/0196-9781(85)90179-2. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y., Kawai Y., Shiosaka S., Takami K., Kiyama H., Hillyard C.J. Coexistence of calcitonin gene-related peptide and substance P-like peptide in single cells of the trigeminal ganglion of the rat: immunohistochemical analysis. Brain Res. 1985;330(1):194–196. doi: 10.1016/0006-8993(85)90027-7. [DOI] [PubMed] [Google Scholar]
- 17.Lawson S.N., Crepps B., Perl E.R. Calcitonin gene-related peptide immunoreactivity and afferent receptive properties of DRG neurones in guinea-pigs. J Physiol. 2002;540(Pt 3):989–1002. doi: 10.1113/jphysiol.2001.013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverman J.D., Kruger L. An interpretation of dental innervation based upon the pattern of calcitonin gene-related peptide (CGRP)-immunoreactive thin sensory axons. Somatosens Res. 1987;5(2):157–175. doi: 10.3109/07367228709144624. [DOI] [PubMed] [Google Scholar]
- 19.Luthman J., Johansson O., Ahlström U., Kvint S. Immunohistochemical studies of the neurochemical markers: CGRP, enkephalin, galanin, gamma-MSH, NPY, PHI, proctolin, PTH, somatostatin, SP, VIP, tyrosine hydroxylase and neurofilament in nerves and cells of the human attached gingiva. Arch Oral Biol. 1988;33(3):149–158. doi: 10.1016/0003-9969(88)90039-8. [DOI] [PubMed] [Google Scholar]
- 20.Kvinnsland I., Kvinnsland S. Changes in CGRP-immunoreactive nerve fibres during experimental tooth movement in rats. Eur J Orthod. 1990;12(3):320–329. doi: 10.1093/ejo/12.3.320. [DOI] [PubMed] [Google Scholar]
- 21.Kimberly C.L., Byers M.R. Inflammation of rat molar pulp and periodontium causes increased calcitonin gene-related peptide and axonal sprouting. Anat Rec. 1988;222(3):289–300. doi: 10.1002/ar.1092220310. [DOI] [PubMed] [Google Scholar]
- 22.Oku R., Satoh M., Fujii N., Otaka A., Yajima H., Takagi H. Calcitonin gene-related peptide promotes mechanical nociception by potentiating release of substance P from the spinal dorsal horn in rats. Brain Res. 1987;403(2):350–354. doi: 10.1016/0006-8993(87)90074-6. [DOI] [PubMed] [Google Scholar]
- 23.Biella G., Panara C., Pecile A., Sotgiu M.L. Facilitatory role of calcitoningene-related peptide (CGRP) on excitation induced by substance P (SP) and noxious stimuli in rat spinal dorsal horn neurons. An iontophoretic study in vivo. Brain Res. 1991;559(2):352–356. doi: 10.1016/0006-8993(91)90024-p. [DOI] [PubMed] [Google Scholar]
- 24.Cady R.J., Glenn J.R., Smith K.M., Durham P.L. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheraland central sensitization. Mol Pain. 2011;7:94. doi: 10.1186/1744-8069-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Felice M., Porreca F. Opiate-induced persistent pronociceptive trigeminal neural adaptations: potential relevance to opiate-induced medication overuse headache. Cephalalgia. 2009;29(12):1277–1284. doi: 10.1111/j.1468-2982.2009.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edvinsson L., Goadsby P.J. Neuropeptides in the cerebral circulation: relevance to headache. Cephalalgia. 1995;15(4):272–276. doi: 10.1046/j.1468-2982.1995.1504272.x. [DOI] [PubMed] [Google Scholar]
- 27.Ashina M., Bendtsen L., Jensen R., Schifter S., Jansen-Olesen I., Olesen J. Plasma levels of calcitonin gene-related peptide in chronic tension-type headache. Neurology. 2000;55:1335–1340. doi: 10.1212/wnl.55.9.1335. [DOI] [PubMed] [Google Scholar]
- 28.Bellamy J.L., Cady R.K., Durham P.L. Salivary levels of CGRP and VIP in rhinosinusitis and migraine patients. Headache. 2006;46(1):24–33. doi: 10.1111/j.1526-4610.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- 29.Juhasz G., Zsombok T., Modos E.A., Olajos S., Jakab B., Nemeth J. NO-induced migraine attack: strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain. 2003;106(3):461–470. doi: 10.1016/j.pain.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Goadsby P.J. Migraine: emerging treatment options for preventive and acute attack therapy. Expert Opin Emerg Drugs. 2006;11(3):419–427. doi: 10.1517/14728214.11.3.419. [DOI] [PubMed] [Google Scholar]
- 31.Hansen J.M., Hauge A.W., Olesen J., Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30(10):1179–1186. doi: 10.1177/0333102410368444. [DOI] [PubMed] [Google Scholar]
- 32.Yisarakun W., Chantong C., Supornsilpchai W., Thongtan T., Srikiatkhachorn A., Reuangwechvorachai P. Up-regulation of calcitonin gene-related peptide in trigeminal ganglion following chronic exposure to paracetamol in a CSD migraine animal model. Neuropeptides. 2015;51:9–16. doi: 10.1016/j.npep.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Karsan N., Goadsby P.J. CGRP mechanism antagonists and migraine management. Curr Neurol Neurosci Rep. 2015;15(5):25. doi: 10.1007/s11910-015-0547-z. [DOI] [PubMed] [Google Scholar]
- 34.Said S.I., Mutt V. Potent peripheral and splanchnic vasodilator peptide from normal gut. Nature. 1970;225(5235):863–864. doi: 10.1038/225863a0. [DOI] [PubMed] [Google Scholar]
- 35.Lorén I., Emson P.C., Fahrenkrug J., Björklund A., Alumets J., Håkanson R. Distribution of vasoactive intestinal polypeptide in the rat and mouse brain. Neuroscience. 1979;4(12):1953–1976. doi: 10.1016/0306-4522(79)90068-x. [DOI] [PubMed] [Google Scholar]
- 36.Said S.I. Vasoactive intestinal polypeptide (VIP): current status. Peptides. 1984;5(2):143–150. doi: 10.1016/0196-9781(84)90197-9. [DOI] [PubMed] [Google Scholar]
- 37.Harmar A.J., Arimura A., Gozes I., Journot L., Laburthe M., Pisegna J.R. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50(2):265–270. [PMC free article] [PubMed] [Google Scholar]
- 38.Kummer W., Heym C. Correlation of neuronal size and peptide immunoreactivity in the guinea-pig trigeminal ganglion. Cell Tissue Res. 1986;245(3):657–665. doi: 10.1007/BF00218569. [DOI] [PubMed] [Google Scholar]
- 39.Lazarov N. Primary trigeminal afferent neuron of the cat: II. Neuropeptide-and serotonin-like immunoreactivity. J Hirnforsch. 1994;35(3):373–389. [PubMed] [Google Scholar]
- 40.Miyata A., Arimura A., Dahl R.R., Minamino N., Uehara A., Jiang L. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164(1):567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 41.Dickson L., Finlayson K. VPAC and PAC receptors: from ligands to function. Pharmacol Ther. 2009;121(3):294–316. doi: 10.1016/j.pharmthera.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Moller K., Zhang Y.Z., Håkanson R., Luts A., Sjölund B., Uddman R. Pituitary adenylate cyclase activating peptide is a sensory neuropeptide: immunocytochemical and immunochemical evidence. Neuroscience. 1993;57(3):725–732. doi: 10.1016/0306-4522(93)90018-b. [DOI] [PubMed] [Google Scholar]
- 43.Tajti J., Uddman R., Möller S., Sundler F., Edvinsson L. Messenger molecules and receptor mRNA in the human trigeminal ganglion. J Auton Nerv Syst. 1999;76(2–3):176–183. doi: 10.1016/s0165-1838(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 44.Lazarov N. Distribution of calcitonin gene-related peptide- and neuropeptide Y-like immunoreactivity in the trigeminal ganglion and mesencephalic trigeminal nucleus of the cat. Acta Histochem. 1995;97(2):213–223. doi: 10.1016/S0065-1281(11)80102-9. [DOI] [PubMed] [Google Scholar]
- 45.Wakisaka S., Takikita S., Sasaki Y., Kato J., Tabata M.J., Kurisu K. Cell size-specific appearance of neuropeptide Y in the trigeminal ganglion following peripheral axotomy of different branches of the mandibular nerve of the rat. Brain Res. 1993;620(2):347–350. doi: 10.1016/0006-8993(93)90179-q. [DOI] [PubMed] [Google Scholar]
- 46.Magnussen C., Hung S.P., Ribeiro-da-Silva A. Novel expression pattern of neuropeptide Y immunoreactivity in the peripheral nervous system in a rat model of neuropathic pain. Mol Pain. 2015;11:31. doi: 10.1186/s12990-015-0029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jancsó G., Hökfelt T., Lundberg J.M., Kiraly E., Halász N., Nilsson G. Immunohistochemical studies on the effect of capsaicin on spinal and medullary peptide and monoamine neurons using antisera to substance P, gastrin/CCK, somatostatin, VIP, enkephalin, neurotensin and 5-hydroxytryptamine. J Neurocytol. 1981;10(6):963–980. doi: 10.1007/BF01258524. [DOI] [PubMed] [Google Scholar]
- 48.Ichikawa H., Schulz S., Höllt V., Sugimoto T. The somatostatin sst2A receptor in the rat trigeminal ganglion. Neuroscience. 2003;120(3):807–813. doi: 10.1016/s0306-4522(03)00364-6. [DOI] [PubMed] [Google Scholar]
- 49.Del Fiacco M., Somatostatin Quartu M. galanin and peptide histidine isoleucine in the newborn and adult human trigeminal ganglion and spinal nucleus: immunohistochemistry, neuronal morphometry and colocalization with substance P. J Chem Neuroanat. 1994;7(3):171–184. doi: 10.1016/0891-0618(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 50.Takeda M., Kadoi J., Takahashi M., Nasu M., Matsumoto S. Somatostatin inhibits the excitability of rat small-diameter trigeminal ganglion neurons that innervate nasal mucosa and project to the upper cervical dorsal horn via activation of somatostatin 2a receptor. Neuroscience. 2007;148(3):744–756. doi: 10.1016/j.neuroscience.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi M., Takeda M., Matsumoto S. Somatostatin enhances tooth-pulp-evoked cervical dorsal horn neuronal activity in the rat via inhibition of GABAergic interneurons. Brain Res Bull. 2014;100:76–83. doi: 10.1016/j.brainresbull.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 52.Skofitsch G., Jacobowitz D.M. Galanin-like immunoreactivity in capsaicin sensitive sensory neurons and ganglia. Brain Res Bull. 1985;15(2):191–195. doi: 10.1016/0361-9230(85)90135-2. [DOI] [PubMed] [Google Scholar]
- 53.Holland P.R., Akerman S., Goadsby P.J. Orexin 1 receptor activation attenuates neurogenic dural vasodilation in an animal model of trigeminovascular nociception. J Pharmacol Exp Ther. 2005;315(3):1380–1385. doi: 10.1124/jpet.105.090951. [DOI] [PubMed] [Google Scholar]
- 54.Holland P.R., Akerman S., Goadsby P.J. Modulation of nociceptive dural input to the trigeminal nucleus caudalis via activation of the orexin 1 receptor in the rat. Eur J Neurosci. 2006;24(10):2825–2833. doi: 10.1111/j.1460-9568.2006.05168.x. [DOI] [PubMed] [Google Scholar]
- 55.Hou M., Uddman R., Tajti J., Edvinsson L. Nociceptin immunoreactivity and receptor mRNA in the human trigeminal ganglion. Brain Res. 2003;964(2):179–186. doi: 10.1016/s0006-8993(02)03927-6. [DOI] [PubMed] [Google Scholar]
- 56.Ichiki T., Kuroishi K.N., Gunjigake K.K., Kobayashi S., Goto T. Neurokinin B activates the formation and bone resorption activity of rat osteoclasts. Neuropeptides. 2011;45(3):239–244. doi: 10.1016/j.npep.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 57.Fukuda A., Goto T., Kuroishi K.N., Gunjigake K.K., Kataoka S., Kobayashi S. Hemokinin-1 competitively inhibits substance P-induced stimulation of osteoclast formation and function. Neuropeptides. 2013;47(4):251–259. doi: 10.1016/j.npep.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Tajti J., Szok D., Majláth Z., Tuka B., Csáti A., Vécsei L. Migraine and neuropeptides. Neuropeptides. 2015;52:19–30. doi: 10.1016/j.npep.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 59.Goto T., Tanaka T. Tachykinins and tachykinin receptors in bone. Microsc Res Technol. 2002;58(2):91–97. doi: 10.1002/jemt.10123. [DOI] [PubMed] [Google Scholar]
- 60.Holton F.A., Holton P. The possibility that ATP is a transmitter at sensory nerve endings. J Physiol. 1953;119(4):50P–51P. [PubMed] [Google Scholar]
- 61.Ohsawa K., Dowe G.H., Morris S.J., Whittaker V.P. The lipid and protein content of cholinergic synaptic vesicles from the electric organ of Torpedo marmorata purified to constant composition: implications for vesicle structure. Brain Res. 1979;161(3):447–457. doi: 10.1016/0006-8993(79)90674-7. [DOI] [PubMed] [Google Scholar]
- 62.Blaschke E., Uvnäs B. Effect of splenic nerve stimulation on the contents of noradrenaline: ATP and sulphomucopolysaccharides in noradrenergic vesicle fractions from the cat spleen. Acta Physiol Scand. 1979;105(4):496–507. doi: 10.1111/j.1748-1716.1979.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 63.Sawada K., Echigo N., Juge N., Miyaji T., Otsuka M., Omote H. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA. 2008;105(15):5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X., Chen Y., Wang C., Huang L.Y. Neuronal somatic ATP release triggers neuron-SGC communication in dorsal root ganglia. Proc Natl Acad Sci USA. 2007;104(23):9864–9869. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Illes P., Nörenberg W. Neuronal ATP receptors and their mechanism of action. Trends Pharmacol Sci. 1993;14(2):50–54. doi: 10.1016/0165-6147(93)90030-n. [DOI] [PubMed] [Google Scholar]
- 66.Ding Y., Cesare P., Drew L., Nikitaki D., Wood J.N.A.T.P. P2X receptors and pain pathways. J Auton Nerv Syst. 2000;81(1–3):289–294. doi: 10.1016/s0165-1838(00)00131-4. [DOI] [PubMed] [Google Scholar]
- 67.Lewis C., Neidhart S., Holy C., North R.A., Buell G., Surprenant A. Coexpression of P2 × 2 and P2 × 3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377(6548):432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- 68.Shinoda M., Kawashima K., Ozaki N., Asai H., Nagamine K., Sugiura Y. P2 × 3 receptor mediates heat hyperalgesia in a rat model of trigeminal neuropathic pain. J Pain. 2007;8(7):588–597. doi: 10.1016/j.jpain.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Staikopoulos V., Sessle B.J., Furness J.B., Jennings E.A. Localization of P2 × 2 and P2 × 3 receptors in rat trigeminal ganglion neurons. Neuroscience. 2007;144(1):208–216. doi: 10.1016/j.neuroscience.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Katagiri A., Shinoda M., Honda K., Toyofuku A., Sessle B.J., Iwata K. SGC P2Y12 receptor in the trigeminal ganglion is involved in lingual neuropathic pain mechanisms in rats. Mol Pain. 2012;8:23. doi: 10.1186/1744-8069-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li J., Vause C.V., Durham P.L. Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res. 2008;1196:22–32. doi: 10.1016/j.brainres.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takeda M., Takahashi M., Matsumoto S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci Biobehav Rev. 2009;33(6):784–792. doi: 10.1016/j.neubiorev.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Shinoda M., Iwata K. Neural communication in the trigeminal ganglion contributes to ectopic orofacial pain. J Oral Biosci. 2013;55(4):165–168. [Google Scholar]
- 74.Scholz J., Woolf C.J. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10(11):1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 75.Ton B.H., Chen Q., Gaina G., Tucureanu C., Georgescu A., Strungaru C. Activation profile of dorsal root ganglia Iba-1 (+) macrophages varies with the type of lesion in rats. Acta Histochem. 2013;115(8):840–850. doi: 10.1016/j.acthis.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 76.Robitaille R. Purinergic receptors and their activation by endogenous purines at perisynaptic glial cells of the frog neuromuscular junction. J Neurosci. 1995;15(11):7121–7131. doi: 10.1523/JNEUROSCI.15-11-07121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stevens B., Fields R.D. Response of Schwann cells to action potentials in development. Science. 2000;287(5461):2267–2271. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- 78.Stevens B., Ishibashi T., Chen J.F., Fields R.D. Adenosine: an activity-dependent axonal signal regulating MAP kinase and proliferation in developing Schwann cells. Neuron Glia Biol. 2004;1(1):23–34. doi: 10.1017/s1740925x04000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rousse I., Robitaille R. Calcium signaling in Schwann cells at synaptic and extra-synaptic sites: active glial modulation of neuronal activity. Glia. 2006;54(7):691–699. doi: 10.1002/glia.20388. [DOI] [PubMed] [Google Scholar]
- 80.Kim C.F., Moalem-Taylor G. Detailed characterization of neuro-immune responses following neuropathic injury in mice. Brain Res. 2011;1405:95–108. doi: 10.1016/j.brainres.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 81.Ren K., Dubner R. Activity-triggered tetrapartite neuron-glial interactions following peripheral injury. Curr Opin Pharmacol. 2016;26:16–25. doi: 10.1016/j.coph.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vega-Avelaira D., Géranton S.M., Fitzgerald M. Differential regulation of immune responses and macrophage/neuron interactions in the DRG in young and adult rats following nerve injury. Mol Pain. 2009;5:70. doi: 10.1186/1744-8069-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eyo U.B., Gu N., De S., Dong H., Richardson J.R., Wu L.J. Modulation of microglial process convergence toward neuronal dendrites by extracellular calcium. J Neurosci. 2015;35(6):2417–2422. doi: 10.1523/JNEUROSCI.3279-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen T., Koga K., Li X.Y., Zhuo M. Spinal microglial motility is independent of neuronal activity and plasticity in adult mice. Mol Pain. 2010;6:19. doi: 10.1186/1744-8069-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trang T., Beggs S., Wan X., Salter M.W. P2 × 4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci. 2009;29(11):3518–3528. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]