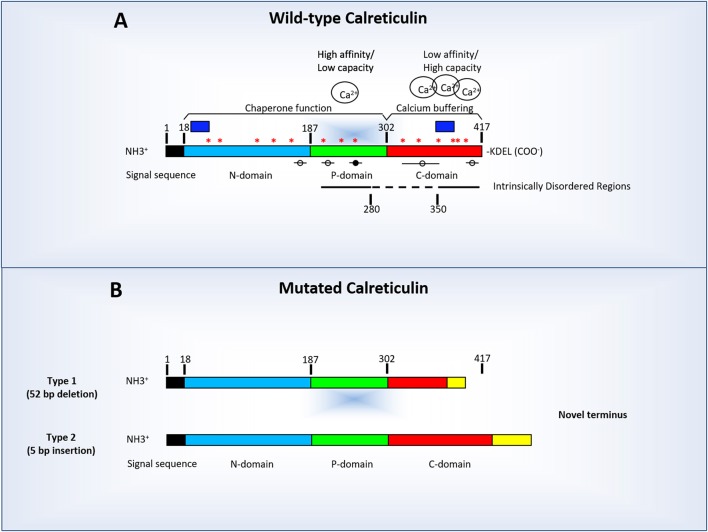
Figure 1.
Linear diagram of human calreticulin indicating its amino-terminal (N-CALR), globular (P-CALR), and carboxy-terminal (C-CALR) domains and the location of the sequences predicted by computer modeling to determine an intrinsically disordered structure (indicated by the straight lines). (A) Normal human CALR and factors which may potentially affect its tertiary structure. In addition to the presence of sequences which determine the intrinsically disordered structure (straight lines), the tertiary structure of CALR is affected by the levels of Ca2+ bound to C-CALR and by binding of other proteins to putative MoRFs sequences (circles and lines). The black circle indicates MoRF3 which has a known putative binding protein. The dashed line indicate the region between AA 260–330 AA predicted by computer modeling to have a stable conformation (i.e., lacking intrinsically disordered regions, binding sites for Ca2+ or MoRFs). The blue boxes indicate the location of the sequences used to raise the commercially available antibodies against human N-CALR (#12238, Cell Signaling, Boston, MA) and C-CALR (sc-6467, Santa Cruz Biotechnology, Santa Cruz, CA). Asterisks indicate putative JAK2-dependent phosphorylation sites. (B) Diagram of the structure of representative Type 1 (deletions) and Type 2 (insertions) CALR mutations found in Philadelphia-negative myeloproliferative disorders. The mutations found in these maladies are all localized in exon 9 encoding the terminal C-region of the protein and encode a truncated (Type 1, top diagram) or elongated (Type 2, bottom diagram) form of C-CALR. In both cases, the mutated C-CALR loses the KDEL motive necessary for translocation in the ER. The mutations induce also loss of sites in the C domain responsible for Ca2+ binding, of three of the putative JAK2-dependent phosphorylation sites and two MoRFs sites (see Table 1 for further details). The mutant proteins may also lose the sequence used to generate the anti-C-CALR antibody commercially available. In this figure, as in rest of the manuscript CALR AA are numbered starting from the first AA of the signal sequence.