Abstract
Singlet O2 is a key reactive oxygen species responsible for photodynamic therapy and is generally recognized to be chemically reactive towards C=C double bonds. Herein, we report the hydroperoxidation/lactonization of α-ethereal C–H bonds by singlet O2 (1Δg) under exceptionally mild conditions, i.e., room temperature and ambient pressure, with modest to high yields (38~90%) and excellent site selectivity. Singlet O2 has been known for > 90 years, but was never reported to be able to react with weakly activated C–H bonds in saturated hydrocarbons. Theoretical calculations indicate that singlet O2 directly inserts into the α-ethereal C–H bond in one step with conservation of steric configuration in products. The current discovery of chemical reaction of singlet oxygen with weakly activated solvent C–H bonds, in addition to physical relaxation pathway, provides an important clue to a 35-year-old unresolved mystery regarding huge variations of solvent dependent lifetime of singlet O2.
Singlet oxygen is known to react with carbon–carbon double bonds. Here, the authors show the unusual functionalization of α-ethereal C–H bonds mediated by singlet oxygen under mild conditions to afford lactones and hydroperoxide products.
Introduction
Singlet oxygen is widely recognized owing to its reactivity towards alkenes1, 2, such as ene reactions, cycloaddition, and heteroatom oxidation1, 2, as well as its involvement in clinical photodynamic therapy treatments of tumors3. However, singlet O2 was never reported to be able to react with C–H bonds in saturated hydrocarbons. In the literature, the lifetimes of singlet oxygen were reported to be strongly dependent on solvents, ranging from 900 μs in tetrachloromethane (CCl4), 20 μs in tetrahydrofuran (THF) to 2 μs in H2O4–7. The large solvent dependence of singlet O2 lifetimes was once attributed to significant overlap between the singlet O2 phosphorescence emission bands and solvent infrared absorptions at ~ 1269 and ~ 1592 nm, as well as solvent perturbation-induced physical deactivation via electronic vibrational coupling4, 5. However, large d-solvent effects (KH/KD ~ 5.8–19.9) on the lifetime of singlet O2 were also observed6, 8, which cannot be well explained by the solvent perturbation model7, 9. When strained hydrocarbons (such as cubanes) were used to quench the excited state of singlet oxygen, the quenching rate is one order faster than that predicted by the solvent C–H bond vibration-induced perturbation model9. There is a large variation and inconsistency between the experimentally measured singlet oxygen lifetimes and theoretically calculated values derived from solvent C–H bond vibration-induced physical perturbation model7–9. It was once found that the short lifetime of singlet oxygen is related to the presence of olefinic impurities in methyl tert-butyl ether (MTBE)10. Detailed analysis shows that in addition to the olefinic impurity quenching process, pure MTBE itself can also deactivate the singlet O2, via physical quenching, with a rate of 2.9 × 103 M−1s−1 11. The impurity quenching mechanism cannot account for the short lifetimes of singlet oxygen in various organic solvents, since not all solvents contain olefinic impurities. To-date, the origin of the huge lifetime variations of singlet O2 in different solvents is still a mystery6–9, 12.
Here, we report that direct oxidative hydroperoxidation of weakly activated α-ethereal C–H bond of aliphatic ether compounds could be achieved with excellent site selectivity and good yields via direct C–H bond insertion and conservation of steric configuration using singlet O2, in situ generated either by photo-sensitization of triplet sensitizers (such as Ir(ppy)2acac, rose bengal or meso-tetraphenylporphyrin, meso-TPP), direct photoexcitation of molecular oxygen using 760 nm or 1270 nm light, or by dark reaction of H2O2 with Na2MoO2 (or HClO). We also demonstrate that the singlet O2-mediated C–H bond functionalization can also be applied to molecules having complicated structures for hydroperoxidation/lactonization at the α-ethereal C–H site. Such a chemical reaction between singlet oxygen and solvent C–H bonds was never considered as a deactivation channel for singlet oxygen and might provide an important clue or explanation for the huge variation of singlet oxygen lifetimes in different solvents.
Results
Chemical reaction of THF with singlet oxygen
In this study, we demonstrate that singlet oxygen can chemically react with C–H bonds of various ethereal solvent molecules, leading to the formation of hydroperoxide and lactone products (Tables 1 and 2). We isolated singlet O2-THF chemical reaction products after photo irradiation of a THF solution in the presence of a singlet O2 photosensitizer, e.g., meso-TPP. THF was freshly distillated prior to all experiments to avoid interference from any possible pre-existing hydroperoxides. Surprisingly, after 2 h of photo irradiation by blue LEDs (460 nm, 40 mW cm−2) at room temperature, formation of 2-hydroperoxyl-THF (2a) and tetrahydrofuran-2(3 H)-one (3a) were observed with a total yield of 27%. The yield was increased to 45% in the presence of a Lewis acid, such as, γ-Al2O3 or Et3B, in the solution (Table 1, entry 1). The hydroperoxidation and lactonization occur exclusively and selectively at the α-ethereal position, and no β-ethereal product was observed. The involvement of singlet O2 in the formation of 2a and 3a was confirmed by several control experiments listed below. Phosphorescence of singlet O2 at ~ 1270 nm was observed. In the absence of photosensitizers, no products and singlet O2 phosphorescence were observed under the same 460 nm LED light irradiation condition. The presence of NaN3, a well-known singlet O2 quencher, markedly suppresses the yields of 2a and 3a. Generation of singlet O2 via a dark reaction of H2O2 with strong oxidants (such as, HOCl or NaMoO4)13 in THF also leads to the formation of the products, 2a and 3a. Addition of olefins to the THF- meso-TPP solution results in the formation of expected singlet O2-ene reaction products and decreases the product yields of 2a and 3a.
Table 1.
Substrate scope of aliphatic ethers
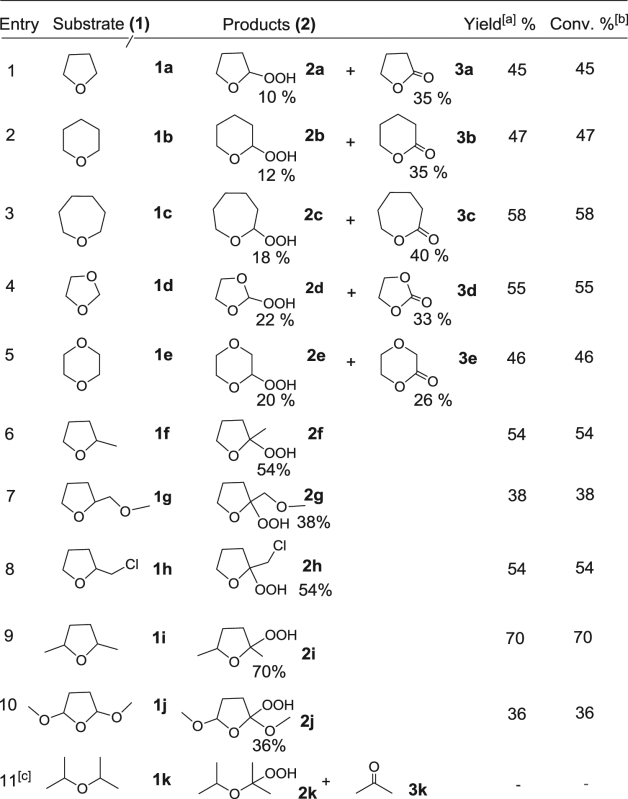
|
Selective α-hydroperoxidation/lactonization of aliphatic cyclic and linear ethers by 1O2. All neat substrates were irradiated by blue LEDs (40 mW cm−2) for 8 h at room temperature in the presence of 1 × 10−5 M of meso-TPP, ~ 1 atm O2 balloon, and the presence of γ-Al2O3. All values reported were based on isolated yields (after removal of unreacted starting substrate)
[a] % of yield was expressed with respect to the amount of starting materials. Beside the listed products, the rest of material is unreacted starting substrate
[b] Conv % = 100%−(% recovered starting substrate)
[c] Yields were not reported due to the high volatility of the products
Table 2.
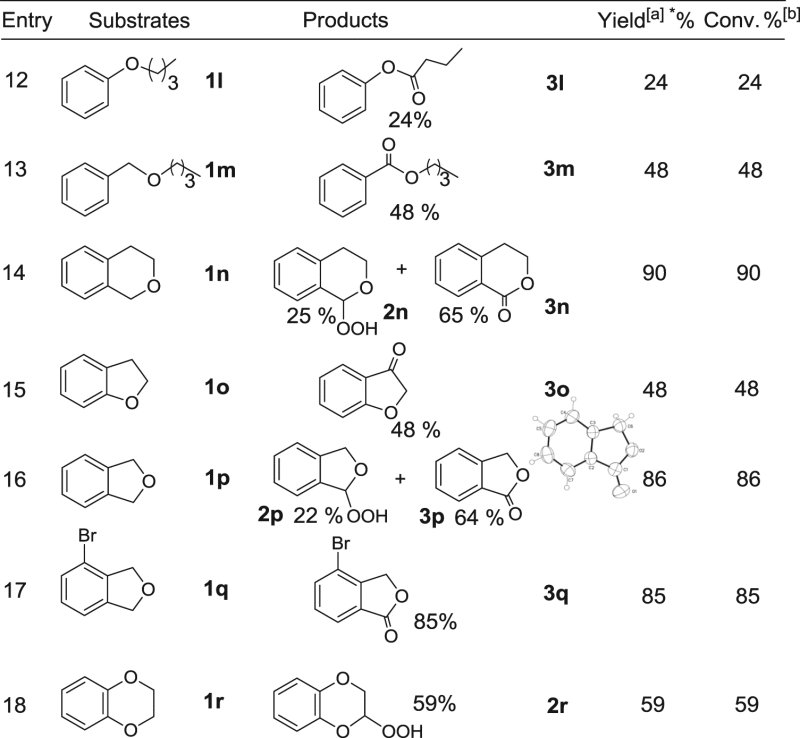
Substrate scope of aromatic ring containing compounds
|
Selective α-hydroperoxidation/lactonization of aromatic cyclic/linear ethers by 1O2. All substrates (0.35 M, in DCM-ACN (9:1)) were irradiated by blue LEDs (40 mW cm−2) for 12 h at room temperature in the presence of 1 × 10−5 M of meso-TPP, ~ 1 atm O2 balloon, and in the presence of γ-Al2O3. All yields were reported based isolated yields (after removal of the unreacted starting substrate)
[a] % of yield was expressed with respect to the amount of starting materials. Beside the listed products, the rest of material is unreacted starting substrate
[b] Conv % = 100%−(% recovered starting substrate)
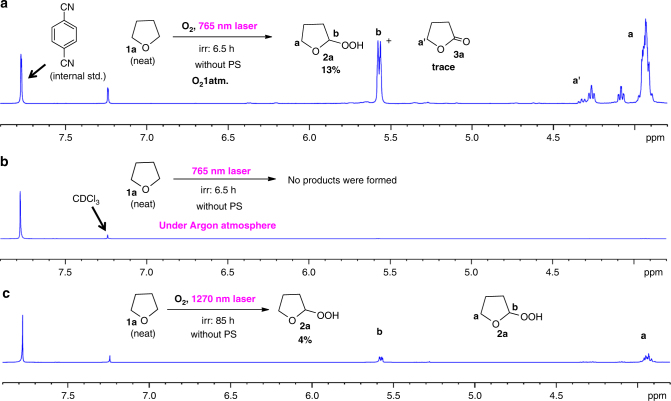
It is reported that singlet oxygen can be generated via direct photo irradiation of molecular oxygen by either 765 nm light14 or 1270 nm NIR light15. In the current system, freshly distillated neat THF was irradiated by 765 nm light (output from a Ti:sapphire laser, 477 mW cm−2, CW mode, 6.5 h) under 1 atm oxygen atmosphere. Figure 1a shows that THF-hydroperoxide and a subsequent lactone decomposition product were observed. These two products were not formed in an argon atmosphere under the same irradiation condition (Fig. 1b). When the THF-O2 solution was irradiated by 1270 nm (output from a diode laser, 3.2 mW cm−2, CW mode, 85 h), the THF-hydroperoxide was formed without co-formation of the lactone product (Fig. 1c). These experiments unambiguously demonstrate that it is singlet oxygen, that reacts with THF to form the THF-hydroperoxide (and/or lactone) product.
Fig. 1.
1H-NMR spectra of photo irradiation products. Products were obtained from direct photoexcitation of molecular oxygen in a neat THF solution under different conditions: a O2 atmosphere, 765 nm, 477 mW cm−2, continuous wave (CW mode), 6.5 h; b Ar atmosphere, 765 nm, 477 mW cm−2, CW mode, 6.5 h; and c O2 atmosphere, 1270 nm, 3.2 mW cm−2, CW mode, 85 h. PS = photosensitizer
The O–O bond strength (34~45 kcal/mol) of hydroperoxides is very weak, and thermal decomposition occurs easily to form hydroxyl radical and O-centered alkoxyl radical16. The hydroxyl radical can abstract a proton from the weakest geminal C–H site, via breakage of a C–H bond (~ 98 kcal/mol), to form a strong O–H bond (~ 110 kcal/mol) and a C=O bond (~ 88 kcal/mol), leading to the formation of corresponding lactone. Overall, such an O–O bond cleavage in hydroperoxide or hydroperoxy acetals is exothermic and thermodynamically favored. Rearrangement of hydroperoxides (via the Hock rearrangement mechanism) or hydroperoxy acetals to form corresponding ketones, esters, or lactones are well documented in the literature11, 17. These rearrangements can be catalyzed by metal ions and Lewis acids18. Rapid explosion often occurs by mistakenly adding metal ions-containing waste water into hydroperoxides-containing organic wastes.
The chemical reaction rate of THF with singlet O2 was determined to be 3.8 × 103 M−1s−1 (see Supplementary Disscussion and Supplementary Fig. 36) by using 1-methylcyclohexene as a competing reactant, of which a reaction rate of 0.16 × 106 M−1s−1 with singlet O2 is known19. The THF reaction rate is compatible with those for poorly reactive olefins, such as, 2-methyl propene (4.4 × 103 M−1s−1)20, and bimolecular quenching rates of singlet O2 by cubanes (103~104 M−1s−1)9. Such a chemical reaction rate corresponds to a singlet O2 lifetime of ~ 21 µs in neat THF, which is also in good agreement with the range of 20~30 µs lifetime of singlet O2 in THF as reported in the literature4–7, 21. In this study, we explicitly demonstrate that chemical reaction of singlet O2 with THF solvent molecules has a quantum yield of 0.93 (see Supplementary Equations 4, 5, and 6), and the chemical reaction rate is one order faster than the solvent collision induced physical relaxation. Therefore, the chemical reaction of singlet oxygen with THF solvent molecule is the dominant deactivation channel responsible for the short lifetime (20 μs) of singlet O2 in THF, when compared to the very long lifetime (900 μs) of singlet O2 in CCl4. Such a chemical deactivation channel was not considered or recognized in the past 35 years to account for short lifetimes of singlet oxygen in various solvents. The observation of the chemical reaction between singlet O2 and α-ethereal C–H bonds of THF is consistent with some previously observed clues that the inverse singlet O2 lifetime (1/τ) is linearly proportional to molar concentrations of solvent C–H bonds5, 6, and negative activation volume observed in high pressure lifetime measurements22.
Synthetic applications
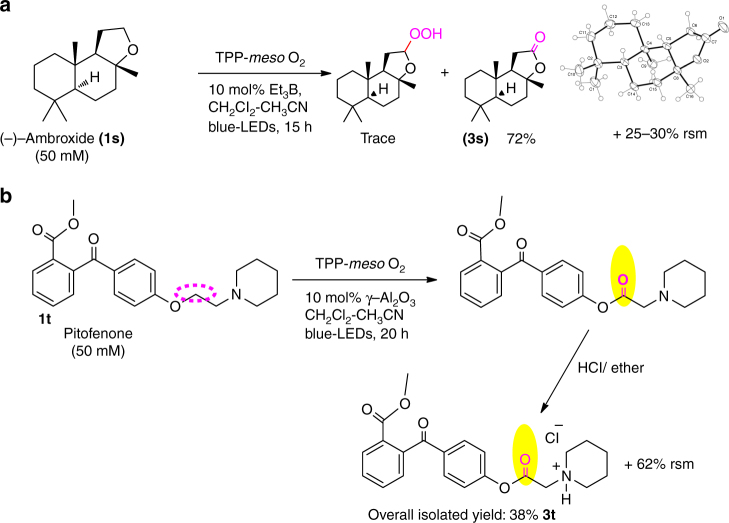
To explore the synthetic application of such a unique singlet O2-mediated α-ethereal C–H bond functionalization, many cyclic/linear aliphatic ether-containing substrates were subjected to photo irradiation in the presence of air and a photosensitizer. Tables 1 and 2 show many aliphatic cyclic and linear ether substrates can react with singlet O2 to form hydroperoxides and/or lactones with yields ranging from 24 to 90% after 8-12 h blue LED light irradiation at room temperature in the presence of a Lewis acid, γ-Al2O3 under 1 atm O2 atmosphere. In the absence of γ-Al2O3, the same products were obtained in lower yields. When the substrates contain both α-methylene C–H and α-methine C–H bonds, the hydroperoxidation occurs selectively and exclusively at the methine C–H bond (Table 1, entries 6–8). Upon co-existence of α-benzylic C–H and α-methylene C–H bonds, the hydroperoxidation occurs selectively and exclusively at the α-benzylic C–H bond (Table 2, entries 14 and 15). To further explore the possibility of using singlet O2 for α-ethereal C–H bond functionalization of ethereal molecules with complex structures, (-)-ambroxide (1s) was photolyzed in a dichloromethane-acetonitrile (9:1) solution in the presence of meso-TPP (1 × 10−5 M) and 10 mol% triethyl borane (Et3B). After 15 h blue LED light irradiation, sclareolide (3s) was obtained with 72% yield (Fig. 2a). The product was confirmed by 1H/13C NMR spectroscopy, mass spectrometry and single crystal X-ray diffraction (see Supplementary Fig. 29 and 30). As expected, lactonization occurs selectively and exclusively at α-ethereal C–H bond of this macrocyclic ether, but not at methyl, methylene or methine C–H bonds. Only trace amounts of hydroperoxide product were observed, which were characterized by 1H-NMR. When pitofenone was subjected to a reaction with singlet O2, an ester was formed selectively at the methylene site adjacent to the ethereal oxygen, but not at those adjacent to tertiary amine site (Fig. 2b).
Fig. 2.
Singlet oxygen reaction with structurally complex substrates. Singlet O2-mediated direct aliphatic α-ethereal C–H oxidative functionalization of substrates: (a) (-)-ambroxide, and (b) pitofenone. Rsm: recovered starting material. The yield refers to the isolated product after workup
Reaction mechanism
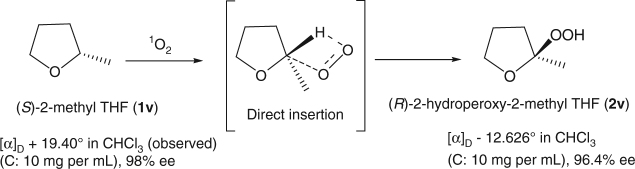
To examine whether the hydroperoxidation of α-ethereal C–H bond by singlet O2 occurs via a single-step direct insertion process or two steps hydrogen atom abstraction followed by radical recombination, chiral (S)-2-methyl THF (1v, ((α)D + 19.40°, 98% ee, see Supplementary Figs. 32–35) was synthesized according the procedure from the literature23, and then subjected to singlet O2 oxidation under standard condition. As expected, hydroperoxidation reaction occurs selectively at the α-methine C–H site. Chiral column separation data (see Supplementary Fig. 32–35) show that the hydroperoxidation products ((α)D −12.626°) contain 98.22% (R)-2-hydroperoxyl-2-methyl THF and 1.78% (S)-2-hydroperoxyl-2-methyl THF, that is, 96.4% ee excess of (R)-2-hydroperoxyl-2-methyl THF, which is close to the 98% ee of the starting chiral (S)-2-methyl THF. The chiral (R)-2-hydroperoxyl-2-methyl THF has the same steric configuration as the starting substrate ((α)D + 19.40°, see Supplementary Figures 32~35)23. The oxidation of C–H to COOH proceeds with at least nearly complete steric retention. The results unambiguously indicate that the hydroperoxidation of α-ethereal C–H bond by singlet O2 occurs via a single-step direct insertion process (Fig. 3).
Fig. 3.
Direct C–H bond insertion and retention of configuration. Mechanistic study for singlet O2-mediated direct aliphatic α-ethereal C–H oxidative functionalization of (S)-2-methyl THF. The optical rotation and ee values are based on experimental measurements (see Supplementary Fig. 32–35)
In addition to the above singlet oxygen direction C–H bond insertion mechanism, the involvements of other species, such as superoxides (via electron transfer from electronically excited photosensitizers to molecular oxygen) or radicals, was also examined. The addition of 25 mol% of KO2, a stable source of superoxide, to THF for 36 h in the dark did not result in the formation of any trace amounts of THF-hydroperoxide or lactone. Therefore, the participation of superoxide in the reaction with THF to form the oxidation products, 2a and 3a, is unambiguously excluded. We have also added 5 mol% (relative to photosensitizer) of a radical trapping reagent, namely, DMPO (5,5-Dimethyl-1-Pyrroline-N-Oxide), into a meso-TPP -THF solution. The total yield of THF-hydroperoxide was reduced slightly from 27% (in the absence of DMPO) to 22% (in the presence of DMPO) after 8 h photo irradiation using 100 W Hg lamp, suggesting that very little (or no) amount of THF-hydroperoxide/lactone was produced via a radical-associated pathway.
Theoretical calculations
To look for theoretical support to the above observed one-step direct insertion process of singlet O2 to the α-ethereal C–H bond, we carried out theoretical calculations at the CAS-MP2(20,14)/6-311 G(p)//CAS(20,14)/6-31 G(p) level of theory24. Figure 4 shows that the excited state energy of singlet O2 at the Frank-Condon geometry (FC) is 25 kcal/mol above the ground state configuration. Two minimum-energy pathways on the singlet excited potential energy surface of oxygen were characterized by optimizing the geometries along path A (the direct insertion of singlet O2 at the α-ethereal C–H bond) and path B (the direct insertion of singlet O2 at the β-ethereal C–H bond), which can lead to the different products (2a, 3a + H2O, and 4a, respectively). The results of our theoretical calculations indicate that CI-1 is 3.0 kcal mol−1 lower in energy than FC, whereas CI-2 is 50 kcal mol−1 above FC. The theoretical evidences demonstrate that the path A is strongly preferred over the path B. The THF-hydroperoxide product 2a can be easily produced during the irradiation of reactants (1a and triplet ground state molecular O2) with light. Moreover, tunneling through the CI-1 point can lead to either the direct insertion product (2a) or the initial ground state reactants. It has to be pointed out that the singlet 2a can revert to the starting materials (1a and triplet ground state molecular O2) in a thermal process on the ground state (Fig. 4). After the minimum 2a on the ground state energy surface is produced, there is another reaction pathway (TS-1), from which one may obtain the final rearrangement products (3a and H2O). The theoretical findings reveal that due to the high excess energy of 51 kcal/mol, resulting from the
Fig. 4.
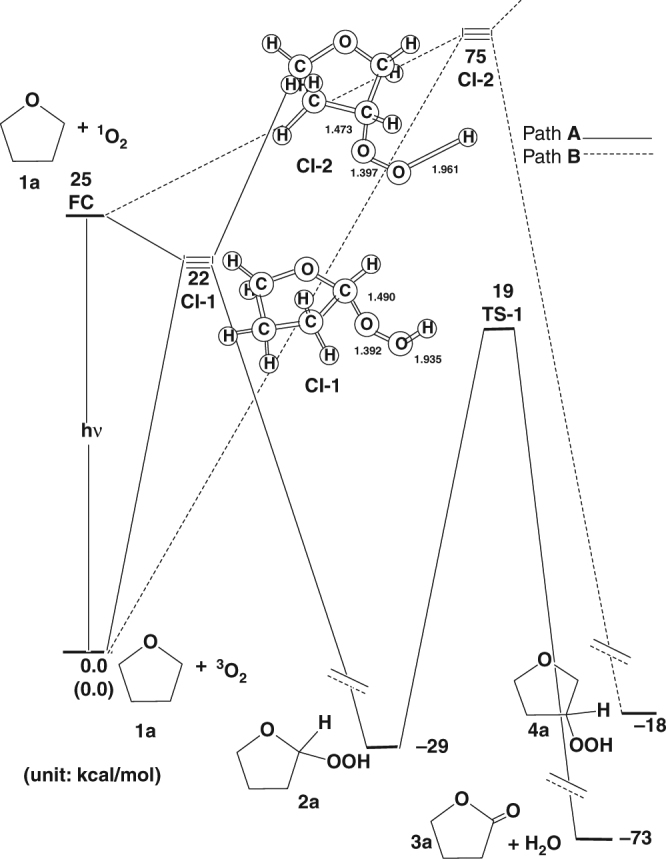
Theoretical calculated energy diagram. Theoretical calculations of direct singlet oxygen insertion into α and β C–H bonds of THF adjacent to the ethereal oxygen were carried out using the CAS-MP2(20,14)/6-311 G(p)//CAS(20,14)/6-31 G(p) method
CI-1 to THF-hydroperoxide 2a, the barrier of 48 kcal mol−1 from 2a to a more stable lactone product, 3a, that can be easily overcome (in the absence of any catalysts). Accordingly, the theoretical investigations suggest that the final lactone product, 3a, can be generated either through the direct irradiation of the initial reactants (1a and triplet ground state molecular O2). The above calculation for conversion of hydroperoxide 2a to lactone 3a is based on the condition in the absence of any catalysts. It has been reported in the literature that metal ions/Lewis acid can catalyze dehydration of hydroperoxide to lactone18. The presence of catalysts may find other pathways with lower activation energies. However, the involvement of catalysts in theoretical calculations is too complicated and beyond the scope of this study.
Discussion
In the current study, the site selectivity of singlet O2 is most probably governed by the C–H bond strength of the α-ethereal C–H bond and the activation energy, rather than by its electrophilicity. From theoretical calculations, it is clear that the α-ethereal C–H bond strength and the energy barrier for direct singlet oxygen insertion at the α C–H bond are much lower than for the β-ethereal C–H bonds. Therefore, direct insertion of singlet oxygen occurs selectively at the α-ethereal C–H bonds, rather than at the more electron-rich β C–H site. The yield of C–H peroxidation products was enhanced in the presence of Lewis acids (such as, Et3B, γ-Al2O3, etc). Coordination of Lewis acids to the ethereal oxygen atom can help withdraw electrons and weaken adjacent α-ethereal C–H bond, favoring attack of α-ethereal C–H bonds by singlet oxygen and higher product yields. This type of site selectivity is unusual for electrophilic oxidants, such as singlet O2. This type of C–H bond site selectivity is similar to direct rhodium carbenoid insertion into C–H bond in alkanes/THF25, but is opposite to what was observed using electrophilic organometallic catalyst and H2O2 as an oxidant, where C–H functionalization occurs at the most remote position (or the most electron-rich site) away from the electron withdrawing directing group26–28. In organic chemistry laboratories, it is commonly known that 6~12 months exposure of THF cans to air and room light will result in the formation of THF-hydroperoxide (but not tetrahydrofuran-2(3 H)-one)29, which was once mistakenly attributed to photoexcitation of a O2-THF ground state charge transfer complex without involving singlet O2 29. Our study shows that this type of hydroperoxide formation commonly observed in ethereal solvents might be due to the chemical reaction of singlet oxygen with solvent molecules, where singlet oxygen was formed via direct 6~12 months room light excitation of molecular oxygen or via photo-sensitization of trace amounts of light absorbing species existing in the solvents.
Overall, we have unambiguously demonstrated that α-ethereal C–H bonds of aliphatic ethers can be selectively functionalized by singlet O2 with moderate to good yields and excellent site selectivity via a one-step direct insertion process with conservation of steric configuration. The C–H bond insertion rate to α-ethereal C–H bond in THF was experimentally determined to be ~ 3.8 × 103 M−1s−1 We believe that such a type of singlet O2 reaction will soon find its wide applications in synthetic chemistry and likely play a role in photodynamic therapy destruction of cancer cells. This study provides important clues to the literature mystery regarding the lifetime variation of singlet oxygen in various solvents by considering the chemical reaction pathway of singlet oxygen with weakly activated solvent C–H bonds.
Methods
General procedure
All reactions were conducted under an oxygen atmosphere and oven-dried glass wares were used. All reactions were conducted using blue LED lights (30 lamps, power density: 40 mW cm−2) or 100 W Hg lamp as a light source without any filter. All solvents were dried according to known methods, and distilled prior to use. Starting materials including photosensitizers were commercially available and used as purchased. NMR spectra were recorded in CDCl3, 1H-NMR at 400 and 600 MHz, 13C NMR at 100 and 150 MHz. Data reported as: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, and b = broad. Specific optical rotation was obtained by JASCO p-2000 polarimeter (serial no: A060361232) with a sodium lamp and are reported as follows: (α)D (c = 10 mg/mL, solvent: CHCl3). For other details of experimental procedures, please refer to the Supplementary Methods.
Data availability
The authors declare that data supporting the findings of this study are available within the paper and the supplementary information file. Supplementary Figs. 29–30 for 3p and 3s can be obtained from the Cambridge Crystallographic Data Centre www.ccdc.cam.ac.uk/data_request/cif on quoting registry no. CCDC 1436061 (3p) and CCDC 1436060 (3s).
Electronic supplementary material
Acknowledgments
The authors are grateful for the financial support from the Ministry of Science and Technology, Taiwan; as well as the use of computer from the National Center for High-Performance Computing.
Author contributions
K.C.H. conceived the idea, designed experiments, and wrote the manuscript. A.S. performed and co-designed experiments and structural identification of products. M.-D.S. carried out theoretical calculation.
Competing interests
The authors declare no competing financial interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at doi:10.1038/s41467-017-01906-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kuo Chu Hwang, Email: kchwang@mx.nthu.edu.tw.
Ming-Der Su, Email: midesu@mail.ncyu.edu.tw.
References
- 1.Singleton DA, et al. Mechanism of ene reactions of singlet oxygen. A two-step no-intermediate mechanism. J. Am. Chem. Soc. 2003;125:1319–1328. doi: 10.1021/ja027225p. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann N. Photochemical reactions as key steps in organic synthesis. Chem. Rev. 2008;108:1052–1103. doi: 10.1021/cr0680336. [DOI] [PubMed] [Google Scholar]
- 3.Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid. Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- 4.Long C, Kearns DR. Selection rules for the intermolecular enhancement of spin forbidden transitions in molecular oxygen. J. Chem. Phys. 1973;59:5729–5736. doi: 10.1063/1.1679927. [DOI] [Google Scholar]
- 5.Hurst JR, Schuster GG. Nonradiative relaxation of singlet oxygen in solution. J. Am. Chem. Soc. 1983;105:5756–5760. doi: 10.1021/ja00356a009. [DOI] [Google Scholar]
- 6.Ogilby PR, Foote CS. Chemistry of singlet oxygen. 34. unexpected solvent deuterium isotope effects on the lifetime of singlet molecular oxygen (lΔg) J. Am. Chem. Soc. 1981;103:1219–1221. doi: 10.1021/ja00395a041. [DOI] [Google Scholar]
- 7.Ogilby PR, Foote CS. Chemistry of singlet oxygen. 42. Effect of solvent, solvent isotopic substitution, and temperature on the lifetime of singlet molecular oxygen (lΔg) J. Am. Chem. Soc. 1983;105:3423–3430. doi: 10.1021/ja00349a007. [DOI] [Google Scholar]
- 8.Ogilby PR, Foote CS. Chemistry of singlet oxygen. 36. Singlet molecular oxygen (IAJ luminescence in solution following pulsed laser excitation. Solvent deuterium isotope effects on the lifetime of singlet oxygen. J. Am. Chem. Soc. 1982;104:2069–2070. doi: 10.1021/ja00371a067. [DOI] [Google Scholar]
- 9.Lancaster JR, et al. Nonradiative deactivation of singlet oxygen (O) by cubane and its derivatives. Org. Lett. 2008;10:5509–5512. doi: 10.1021/ol802113j. [DOI] [PubMed] [Google Scholar]
- 10.Boneva M, Ivanov SK, Oliveros E, Braun AM. Singlet oxygen quenching by tertiary alkyl ethers. J. Photochem. Photobiol. A: Chem. 1992;68:343–351. doi: 10.1016/1010-6030(92)85243-N. [DOI] [Google Scholar]
- 11.Yaremenko IA, Vil VA, Demchuk DV, Terent’ev AO. Rearrangements of organic peroxides and related processes. Beilstein. J. Org. Chem. 2016;12:1647–1748. doi: 10.3762/bjoc.12.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweitzer C, Schmidt R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 2003;103:1685–1757. doi: 10.1021/cr010371d. [DOI] [PubMed] [Google Scholar]
- 13.Adam W, Kazakov DV, Kazakov VP. Singlet-Oxygen chemiluminescence in peroxide reactions. Chem. Rev. 2005;105:3371–3387. doi: 10.1021/cr0300035. [DOI] [PubMed] [Google Scholar]
- 14.Bregnhøj M, Blázquez-Castro A, Westberg M, Breitenbach T, Ogilby PR. Direct 765 nm optical excitation of molecular oxygen in solution and in single mammalian cells. J. Phys. Chem. B. 2015;119:5422–5429. doi: 10.1021/acs.jpcb.5b01727. [DOI] [PubMed] [Google Scholar]
- 15.Jockusch S, et al. Singlet molecular oxygen by direct excitation. Photochem. Photobiol. Sci. 2008;7:235–239. doi: 10.1039/B714286B. [DOI] [PubMed] [Google Scholar]
- 16.Bach RD, Ayala PY, Schlegel HB. A reassessment of the bond dissociation energies of peroxides. An ab Initio study. J. Am. Chem. Soc. 1996;118:12758–12765. doi: 10.1021/ja961838i. [DOI] [Google Scholar]
- 17.Fisher TJ, Dussault PH. Fragmentation of chloroperoxides: hypochlorite-mediated dehydration of hydroperoxyacetals to esters. Tetrahedron. Lett. 2010;51:5615–5617. doi: 10.1016/j.tetlet.2010.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin SS, Gurol MD. Catalytic decomposition of hydrogen peroxide on iron oxide: kinetics, mechanism, and implications. Environ. Sci. Technol. 1998;32:1417–1423. doi: 10.1021/es970648k. [DOI] [Google Scholar]
- 19.Frimer, A. A & Stephenson, L. M. in Singlet O2: Physical-Chemical Aspects Part 1 Vol. 2 (ed. Frimer, A. A.) Ch. 3 (CRC Press Inc., 1985).
- 20.Hurst JR, Schuster GB. Ene reaction of singlet oxygen: an entropy-controlled process determines the reaction rate. J. Am. Chem. Soc. 1982;104:6854–6856. doi: 10.1021/ja00388a097. [DOI] [Google Scholar]
- 21.Rodgers MJA. Solvent-induced deactivation of singlet oxygen: additivity relationships in nonaromatic solvents. J. Am. Chem. Soc. 1983;105:6201–6205. doi: 10.1021/ja00358a001. [DOI] [Google Scholar]
- 22.Okamoto M, Tanaka F, Teranishi H. Pressure effect on the lifetime of singlet oxygen in solutions. J. Phys. Chem. 1990;94:669–672. doi: 10.1021/j100365a030. [DOI] [Google Scholar]
- 23.Keinan E, Seth KK, Lamed R. Organic synthesis with enzymes. 3. TBADH-catalyzed reduction of chloro ketones. Total synthesis of (+)-(S,S)-(cis-6- methyltetrahydropyran-2-yl)acetic acid: a civet constituent. J. Am. Chem. Soc. 1986;108:3474–3480. doi: 10.1021/ja00272a051. [DOI] [Google Scholar]
- 24.Su MD. CASCSF study on the photochemical transposition reactions of pyrazines. J. Phys. Chem. A. 2006;110:9420–9428. doi: 10.1021/jp060995t. [DOI] [PubMed] [Google Scholar]
- 25.Davies HML, Hansen T, Churchill MR. Catalytic asymmetric C−H activation of alkanes and tetrahydrofuran. J. Am. Chem. Soc. 2000;122:3063–3070. doi: 10.1021/ja994136c. [DOI] [Google Scholar]
- 26.Chen MS, White MC. A predictably selective aliphatic C–H oxidation reaction for complex molecule synthesis. Science. 2007;318:783–787. doi: 10.1126/science.1148597. [DOI] [PubMed] [Google Scholar]
- 27.Chen MS, White MC. Combined effects on selectivity in Fe-Catalyzed methylene oxidation. Science. 2010;327:566–571. doi: 10.1126/science.1183602. [DOI] [PubMed] [Google Scholar]
- 28.Newhouse T, Baran PS. If C–H bonds could talk: selective C–H bond oxidation. Angew. Chem. Int. Ed. 2011;50:3362–3374. doi: 10.1002/anie.201006368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sstenberg VI, Wang CT, Kulevsky N. Photochemical oxidations. III. Photochemical and thermal behavior of α-hydroperoxytetrahydrofuran and its implications concerning the mechanism of photooxidation of ethers. J. Org. Chem. 1970;35:1774–1777. doi: 10.1021/jo00831a012. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the paper and the supplementary information file. Supplementary Figs. 29–30 for 3p and 3s can be obtained from the Cambridge Crystallographic Data Centre www.ccdc.cam.ac.uk/data_request/cif on quoting registry no. CCDC 1436061 (3p) and CCDC 1436060 (3s).