Abstract
AIM
To evaluate the association between mortality-to-incidence ratios (MIRs) and health disparities.
METHODS
In this study, we used the GLOBOCAN 2012 database to obtain the cancer incidence and mortality data for 57 countries, and combined this information with the World Health Organization (WHO) rankings and total expenditures on health/gross domestic product (e/GDP). The associations between variables and MIRs were analyzed by linear regression analyses and the 57 countries were selected according to their data quality.
RESULTS
The more developed regions showed high gastric cancer incidence and mortality crude rates, but lower MIR values than the less developed regions (0.64 vs 0.80, respectively). Among six continents, Oceania had the lowest (0.60) and Africa had the highest (0.91) MIR. A good WHO ranking and a high e/GDP were significantly associated with low MIRs (P = 0.001 and P = 0.001, respectively).
CONCLUSION
The MIR variation for gastric cancer would predict regional health disparities.
Keywords: Gastric cancer, Mortality, Incidence, Mortality-to-incidence ratio, Gross domestic product, Expenditure, World Health Organization
Core tip: The mortality-to-incidence ratios (MIRs), defined as the ratio of the crude rate of mortality to the incidence, could reflect the clinical outcomes of disease. A total of 57 countries was included in this analysis to evaluate the association between MIR and health care disparities. The results showed the more developed regions had high gastric cancer incidence and mortality, but lower MIR than the less developed regions. Otherwise, good World Health Organization ranking and high total expenditures on health/gross domestic product were significantly associated with low MIRs. Therefore, the MIR variation for gastric cancer could predict regional health disparities.
INTRODUCTION
Gastric cancer was the leading cause of cancer mortality worldwide until the 1990s[1], but its incidence has since declined, especially in the developed world. Nevertheless, gastric cancer remains one of the most prevalent cancers in the world[2,3]. Gastric cancer is a multi-factorial disease, having a clear relationship with environmental risks, dietary habits, food storage, Helicobacter pylori infection, and geographic region[4-8]. Gastric cancer is more common in developing countries than in developed countries, and it occurs more frequently in men than in women[7,8].
Previous ethnic and migration studies have indicated that early exposure to environmental factors has a greater influence on the mortality and incidence rates of gastric cancer than is found for genetic factors[9,10]. Modern studies have also explored the mechanism of how Helicobacter pylori infection affects gastric cancer development in cancer stem cell lines and animal models[11,12]. Several recent large-scale database observational studies have documented the incidence of gastric cancer among patients with gastric precancerous lesions in western countries[13,14]. The early detection of precancerous lesions, such as atrophic gastritis, intestinal metaplasia and dysplasia, by esophagogastroduodenoscopy (EGD) and further endoscopic or surgical resection are helping to decrease the progression to malignancy[15] and therefore the morbidity and mortality associated with gastric cancer.
As already mentioned, the mortality rates due to gastric cancer have shown a steady decline globally, but regional differences are evident. Previous studies have demonstrated an annual percent decrease in gastric mortality rate of around 3% to 4% for European countries, the United States, the Republic of Korea, Japan and Australia between 1980 and 2005[16]. A 2% annual percent decrease was noted in gastric mortality rate among major Latin American countries and a less dramatic decline was observed in China[2]. This decline is at least in part due to the introduction of EGD, an important tool for early detection of gastric cancer, and even precancerous lesions. A recent Japanese study concluded that EGD screening was more powerful than either radiographic or photofluorography screening, and reduced the mortality rate from gastric cancer by 57%[17].
All these declines in gastric cancer prevalence suggest that the health care system may be able to improve precancerous lesion detection, early cancer detection, and treatment of gastric cancer to provide further declines in mortality. We therefore considered that the mortality-to-incidence (MIR) ratio for gastric cancer should be low in countries with good health care systems, in agreement with recent findings on prostate cancer[18-21]. The aim of the present study was therefore to clarify the association between human development, the World Health Organization (WHO) ranking, region, total expenditure on health/gross domestic product (GDP; e/GDP), life expectancy, and crude rates of incidence and mortality for gastric cancer in different countries. Our results provide a comprehensive overview of gastric cancer MIRs and health disparities in various countries across the globe.
MATERIALS AND METHODS
The data acquisition was described previously[18]. In brief, the cancer epidemiological data were gathered from the GLOBOCAN 2012 database maintained by the International Agency for Research on Cancer (https://www.iarc.fr/). The WHO rankings of countries were obtained from the World’s Health Systems of WHO. The e/GDP and life expectancies for 2012 were obtained from the World Health Statistics 2015.
Data for 184 countries were obtained from the GLOBOCAN 2012 database. Among these 184 countries, 22 were excluded from the study due to a lack of WHO ranking data. We excluded a further 105 countries due to the availability of mortality/incidence data mentioned in GLOBOCAN 2012 database (rankings E to G for incidence or rankings 4 to 6 for mortality were excluded). This resulted in 57 countries being further analyzed in this study. The MIR is defined as the ratio of the crude rate of mortality and the crude rate of incidence[21].
The methods used for statistical analyses were described previously[18]. Associations between the MIRs and variants among countries were estimated by linear regression. R-squared changes and analysis of variance (ANOVA) were determined using SPSS statistical software version 15.0 (SPSS, Inc., Chicago, IL, United States). P value < 0.05 of a two-sided test were considered statistically significant. Scatter plots were generated using Microsoft Excel 2010.
RESULTS
Incidence and mortality rates of gastric cancer according to regions
We examined the global trends in gastric cancer by analyzing the incidence and mortality numbers and rates according to different regions across the globe. The results are summarized in Table 1. The worldwide MIR is 0.76 and it is higher in less developed regions than in more developed regions (0.80 vs 0.64). The WHO values indicate that the Western Pacific region has the highest incidence and mortality for gastric cancer, regardless of whether this is based on the number, crude rate, or age-standardized rate (ASR) (Table 1). However, this region has the lowest MIR, at 0.72, among the six WHO regions. The highest MIR is reported for the WHO South-East Asia region (0.92). At a continent level, Africa has the lowest rate of incidence but has the highest MIR (0.91). The lowest MIR is found in North America.
Table 1.
Case number, rate and mortality-to-incidence ratio of the incidence and mortality of gastric cancer according to region
| Region |
Number |
Crude rate |
Age-standardized rate |
Mortality-to-incidence ratio1 | |||
| Incidence | Mortality | Incidence | Mortality | Incidence | Mortality | ||
| World | 951594 | 723073 | 13.5 | 10.2 | 12.1 | 8.9 | 0.76 |
| Development | |||||||
| More developed regions | 274509 | 174756 | 22.0 | 14.0 | 10.6 | 6.4 | 0.64 |
| Less developed regions | 677085 | 548317 | 11.7 | 9.4 | 12.7 | 10.2 | 0.80 |
| WHO region categories | |||||||
| WHO Africa region | 19110 | 17589 | 2.2 | 2.0 | 4.0 | 3.7 | 0.91 |
| WHO Americas region | 85354 | 65130 | 8.9 | 6.8 | 6.9 | 5.1 | 0.76 |
| WHO East Mediterranean region | 23454 | 20789 | 3.8 | 3.3 | 5.5 | 4.9 | 0.87 |
| WHO Europe region | 161846 | 126315 | 17.9 | 14.0 | 10.0 | 7.4 | 0.78 |
| WHO South-East Asia region | 90558 | 83249 | 4.9 | 4.5 | 5.7 | 5.3 | 0.92 |
| WHO Western Pacific region | 571139 | 409897 | 31.0 | 22.2 | 22.8 | 15.7 | 0.72 |
| Continent | |||||||
| Africa | 23806 | 21801 | 2.2 | 2.0 | 3.8 | 3.5 | 0.91 |
| Latin America and Caribbean | 60852 | 51435 | 10.1 | 8.5 | 9.7 | 8.1 | 0.84 |
| Northern America | 24502 | 13695 | 7.0 | 3.9 | 4.0 | 2.1 | 0.56 |
| Asia | 699954 | 527074 | 16.5 | 12.4 | 15.8 | 11.7 | 0.75 |
| Europe | 139667 | 107360 | 18.8 | 14.5 | 9.4 | 6.9 | 0.77 |
| Oceania | 2813 | 1708 | 7.5 | 4.5 | 5.1 | 3.0 | 0.60 |
The percentage in the ratio of the crude rate of mortalities and the crude rate of incidences.
WHO ranking and e/GDP were significantly associated with the MIRs for gastric cancer
We conducted a further comparison of the differences in epidemiology among countries by analyzing the 57 selected countries (Table 2). The e/GDP ranged from 2.7% (Oman) to 17.0% (United States of America) with mean ± standard deviation of 8.0% ± 2.6%. Japan had the longest life expectancy (84 years) and the Republic of South Africa had the shortest (60 years). Japan had the highest crude rates for gastric cancer, at 85.3 and 41.4 for incidence and mortality respectively. The MIR of Japan, at 0.49, was the fourth lowest value among the 57 countries. The lowest MIR was found for the Republic of Korea (0.34), which had the highest gastric cancer incidence in terms of ASR (41.8). Among these countries, five have MIR values greater than or equal to 0.90, including Fiji (1.05), Ecuador (0.94), Mauritius (0.92), Chile (0.91) and Egypt (0.90).
Table 2.
World Health Organization rankings, total expenditure on health/ gross domestic product, life expectancy, gastric cancer incidence, mortality and mortality-to-incidence ratio for gastric cancer in selected countries
| Country | Ranking | Total expenditure on health/GDP, % | Life expectancy |
Number |
Crude rate |
Age-standardized rate |
Mortality-to-incidence ratio1 | |||
| Incidence | Mortality | Incidence | Mortality | Incidence | Mortality | |||||
| France | 1 | 11.6 | 82 | 6507 | 4412 | 10.3 | 7 | 4.7 | 2.9 | 0.68 |
| Italy | 2 | 9.2 | 83 | 13001 | 9917 | 21.3 | 16.3 | 8.2 | 5.6 | 0.77 |
| Malta | 5 | 8.7 | 81 | 68 | 33 | 16.2 | 7.9 | 8.0 | 3.3 | 0.49 |
| Singapore | 6 | 4.2 | 83 | 647 | 431 | 12.3 | 8.2 | 8.2 | 5.3 | 0.67 |
| Spain | 7 | 9.3 | 83 | 7810 | 5389 | 16.7 | 11.5 | 7.8 | 4.9 | 0.69 |
| Oman | 8 | 2.7 | 76 | 79 | 68 | 2.7 | 2.3 | 5.3 | 4.7 | 0.85 |
| Austria | 9 | 11.1 | 81 | 1314 | 853 | 15.6 | 10.1 | 6.8 | 4.0 | 0.65 |
| Japan | 10 | 10.3 | 84 | 107898 | 52326 | 85.3 | 41.4 | 29.9 | 12.4 | 0.49 |
| Norway | 11 | 9.3 | 82 | 475 | 311 | 9.6 | 6.3 | 4.6 | 2.8 | 0.66 |
| Portugal | 12 | 9.9 | 81 | 3018 | 2285 | 28.2 | 21.4 | 13.1 | 9.0 | 0.76 |
| Iceland | 15 | 9.0 | 82 | 28 | 18 | 8.5 | 5.5 | 5.0 | 2.9 | 0.65 |
| Luxembourg | 16 | 7.2 | 82 | 67 | 32 | 12.8 | 6.1 | 7.6 | 3.0 | 0.48 |
| Netherlands | 17 | 12.7 | 81 | 1953 | 1391 | 11.7 | 8.3 | 5.6 | 3.7 | 0.71 |
| United Kingdom | 18 | 9.3 | 81 | 6684 | 4534 | 10.6 | 7.2 | 4.7 | 2.9 | 0.68 |
| Ireland | 19 | 8.9 | 81 | 487 | 325 | 10.6 | 7.1 | 6.5 | 4.2 | 0.67 |
| Switzerland | 20 | 11.4 | 83 | 683 | 485 | 8.8 | 6.3 | 4.2 | 2.6 | 0.72 |
| Belgium | 21 | 10.9 | 80 | 1417 | 962 | 13.1 | 8.9 | 5.8 | 3.5 | 0.68 |
| Colombia | 22 | 6.8 | 78 | 5897 | 4981 | 12.4 | 10.5 | 13.4 | 11.2 | 0.85 |
| Sweden | 23 | 9.6 | 82 | 811 | 635 | 8.5 | 6.7 | 3.7 | 2.7 | 0.79 |
| Cyprus | 24 | 7.3 | 82 | 94 | 72 | 8.3 | 6.4 | 5.4 | 4.0 | 0.77 |
| Germany | 25 | 11.3 | 81 | 16015 | 9714 | 19.5 | 11.8 | 7.8 | 4.3 | 0.61 |
| Israel | 28 | 7.4 | 82 | 777 | 516 | 10.1 | 6.7 | 7.1 | 4.5 | 0.66 |
| Canada | 30 | 10.9 | 82 | 3342 | 1937 | 9.6 | 5.6 | 4.9 | 2.7 | 0.58 |
| Finland | 31 | 9.1 | 81 | 641 | 479 | 11.9 | 8.9 | 5.2 | 3.7 | 0.75 |
| Australia | 32 | 8.9 | 83 | 2049 | 1135 | 8.9 | 5.0 | 4.8 | 2.5 | 0.56 |
| Chile | 33 | 7.3 | 80 | 3712 | 3371 | 21.3 | 19.3 | 15.6 | 13.8 | 0.91 |
| Denmark | 34 | 11.0 | 80 | 625 | 351 | 11.2 | 6.3 | 5.6 | 2.9 | 0.56 |
| Costa Rica | 36 | 10.1 | 79 | 874 | 612 | 18.2 | 12.8 | 17.3 | 12 | 0.70 |
| United States | 37 | 17.0 | 79 | 21155 | 11758 | 6.7 | 3.7 | 3.9 | 2.0 | 0.55 |
| Slovenia | 38 | 9.4 | 80 | 468 | 335 | 22.9 | 16.4 | 10.4 | 6.8 | 0.72 |
| Cuba | 39 | 8.6 | 78 | 1126 | 916 | 10.0 | 8.1 | 5.9 | 4.6 | 0.81 |
| New Zealand | 41 | 10.2 | 82 | 393 | 240 | 8.8 | 5.4 | 5.2 | 2.9 | 0.61 |
| Bahrain | 46 | 4.4 | 77 | 29 | 21 | 2.1 | 1.5 | 3.9 | 3.5 | 0.71 |
| Thailand | 47 | 4.5 | 75 | 2841 | 2286 | 4.1 | 3.3 | 3.1 | 2.5 | 0.80 |
| Czech Republic | 48 | 7.5 | 78 | 1595 | 1099 | 15.1 | 10.4 | 7.4 | 4.9 | 0.69 |
| Malaysia | 49 | 4.0 | 74 | 1900 | 873 | 6.5 | 3.0 | 7.8 | 3.6 | 0.46 |
| Poland | 50 | 6.8 | 77 | 6105 | 5197 | 15.9 | 13.6 | 8.4 | 7.0 | 0.86 |
| Jamaica | 53 | 5.6 | 74 | 269 | 229 | 9.7 | 8.3 | 9.1 | 7.1 | 0.86 |
| South Korea | 58 | 7.6 | 82 | 31269 | 10746 | 64.4 | 22.1 | 41.8 | 13 | 0.34 |
| Philippines | 60 | 4.4 | 69 | 2415 | 2043 | 2.5 | 2.1 | 3.8 | 3.3 | 0.84 |
| Slovakia | 62 | 8.1 | 76 | 901 | 633 | 16.4 | 11.6 | 9.6 | 6.5 | 0.71 |
| Egypt | 63 | 4.9 | 71 | 1789 | 1584 | 2.1 | 1.9 | 2.5 | 2.3 | 0.90 |
| Uruguay | 65 | 8.6 | 77 | 577 | 514 | 17.0 | 15.2 | 10 | 8.4 | 0.89 |
| Trinidad and Tobago | 67 | 5.5 | 71 | 67 | 53 | 5.0 | 3.9 | 4.4 | 3.4 | 0.78 |
| Belarus | 72 | 5.0 | 72 | 2961 | 2495 | 31.1 | 26.2 | 18.8 | 15.3 | 0.84 |
| Lithuania | 73 | 6.7 | 74 | 867 | 668 | 26.3 | 20.3 | 13.8 | 10.2 | 0.77 |
| Argentina | 75 | 6.8 | 76 | 3738 | 3273 | 9.1 | 8.0 | 6.7 | 5.7 | 0.88 |
| Estonia | 77 | 5.9 | 77 | 370 | 286 | 27.6 | 21.3 | 13.8 | 9.7 | 0.77 |
| Ukraine | 79 | 7.5 | 71 | 11373 | 9216 | 25.3 | 20.5 | 14.3 | 11.6 | 0.81 |
| Mauritius | 84 | 4.8 | 74 | 121 | 112 | 9.2 | 8.5 | 8 | 7.4 | 0.92 |
| Fiji | 96 | 4.0 | 70 | 18 | 19 | 2.1 | 2.2 | 2.4 | 2.6 | 1.05 |
| Bulgaria | 102 | 7.4 | 75 | 1664 | 1354 | 22.5 | 18.3 | 10.3 | 8.3 | 0.81 |
| Latvia | 105 | 5.9 | 74 | 640 | 484 | 28.6 | 21.7 | 14.3 | 10.2 | 0.76 |
| Ecuador | 111 | 6.4 | 76 | 2401 | 2262 | 16.2 | 15.2 | 16.9 | 15.5 | 0.94 |
| Brazil | 125 | 9.5 | 75 | 19690 | 16077 | 9.9 | 8.1 | 9.2 | 7.4 | 0.82 |
| Russian Federation | 130 | 6.5 | 69 | 38417 | 32854 | 26.9 | 23 | 16 | 13.1 | 0.86 |
| South Africa | 175 | 8.9 | 60 | 2029 | 1529 | 4.0 | 3.0 | 5.1 | 3.9 | 0.75 |
The percentage in the ratio of the crude rate of mortalities and the crude rate of incidences.
We also analyzed the association between the rates of incidence/mortality and the WHO ranking or e/GDP (SF1 and SF2). The results showed no significant association, except for the WHO ranking and the ASR of mortality (P = 0.005, SF2D). However, the use of the MIR for analyses revealed significant associations for both the WHO ranking and e/GDP and the MIR of the 57 countries (R2 = 0.195, P = 0.001; R2 = 0.195, P = 0.001, respectively, Figure 1).
Figure 1.
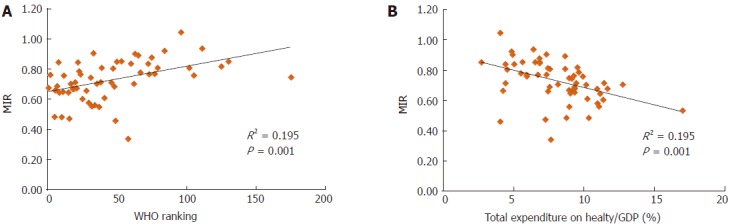
The (A) World Health Organization rankings and (B) total expenditures on health/gross domestic product are significantly associated with MIR in gastric cancer.
DISCUSSION
In this study, we analyzed the correlation of the incidence, mortality and MIRs for gastric cancer with WHO rankings and e/GDP. The MIR, which was calculated as the ratio of the crude rate of mortality and the crude rate of incidence, is regarded as an important marker for cancer care disparities. The crude rates of incidence and mortality, which our results showed were higher in Japan and Korea, are similar to those reported previously[22]. The incidence of gastric cancer can be influenced by environmental hygiene, food storage, diet habits, ethnicity, geographic regions, and, most importantly, age[23].
Otherwise, in countries with high incidence of gastric cancer, more frequent survey or detection of cancer is performed. This might result in more cases detected in early stage and contribute to good clinical outcome. It is also observed in this database that countries with higher incidence of gastric cancer have lower MIR compared with those with lower incidence (crude rate vs MIR: R2 = 0.104, P = 0.015; case number vs MIR: MIR: R2 = 0.078, P = 0.035). Otherwise, a better WHO ranking and a higher e/GDP were correlated linearly with a longer life expectancy (R2 = 0.0.689, P < 0.001; R2 = 0.248, P < 0.001 respectively). This could explain the lack of a significant association between the WHO rankings, e/GDP, and incidence of gastric cancer in our analysis.
The mortality rates for gastric cancer can be reduced by screening programs, early endoscopic detection and management, surgical intervention availability[24,25], and the capability for chemotherapy or targeted therapy[26]. This may be why the ASR of mortality for gastric cancer was correlated with the WHO ranking, but had no significant correlation with total e/GDP. Previous data have shown that MIRs are lower in areas with better health care, and the present study shows that MIRs are also significantly lower in countries with better WHO rankings, with higher e/GDP, and in more developed regions. For the sex difference in the MIR and the health care disparities, previous study has shown that female patients with bladder cancer have higher MIR compared with male patients with bladder cancer[27]. However, unlike bladder cancer, there is no significant association in gastric cancer.
The limitations of this study include the fact that many countries are not participants in the WHO, and many of these countries are located in the least developed areas of the world. This limitation could influence the impact of e/GDP on gastric cancer incidence. Second, the use of the WHO rankings and e/GDP to represent the health care disparities of a country is not particularly specific. We were also unable to analyze ethnicity and national health insurance issues in our study. The reason why only the ASR mortality rate had a significant correlation with WHO rankings needs further investigation.
Our study indicates that gastric cancer has a higher incidence, mortality, and MIR value in less developed regions. Although the incidence, mortality, and MIR values are low in more developed regions, some differences were evident between the geographic regions; for example, the ASR incidence (9.4 vs 15.8 vs 3.8) and mortality (6.9 vs 11.7 vs 3.5) were higher in Europe and Asia than in Africa. The MIRs are generally lower in the more developed continents, as exemplified by North America, which showed the lowest MIR (0.56) and Africa, which showed the highest (0.91).
In conclusion, MIRs showed a significant correlation with WHO rankings and e/GDP in our analysis and we believe that this finding reflects the health care disparities of different countries. Our study provides a valuable documentation of the MIR and its relationship to the global geographic distribution of gastric cancer in 57 countries worldwide.
COMMENTS
Background
The mortality-to-incidence ratios (MIRs), defined as the ratio of the crude rate of mortality to the incidence, reflects the clinical outcomes of cancer patients. The MIRs of colorectal and prostate cancers are associated with health disparities, but similar associations between the MIRs for gastric cancer and health disparities among different countries have never been investigated.
Research frontiers
A total of 57 countries was included in this analysis. The more developed regions showed high gastric cancer incidence and mortality, but lower MIR than the less developed regions. Otherwise, good World Health Organization (WHO) ranking and high total expenditures on health/gross domestic product (e/GDP) were significantly associated with low MIRs.
Innovations and breakthroughs
The MIR variation for gastric cancer could predict regional health disparities.
Applications
The gastric cancer MIR could be used to evaluate the health disparities and ranking of countries.
Terminology
MIRs showed a significant correlation with WHO rankings and e/GDP in 57 countries, which reflects the health care disparities of different countries. Our study provides valuable documentation of the MIR and its relationship to the global geographic distribution of gastric cancer worldwide.
Peer-review
The novelty of this manuscript is good, and the result can help to explain the research purpose.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: There was no conflict of interest for all of the authors.
Data sharing statement: All data are available as mentioned in the section of Materials and Methods.
Peer-review started: June 2, 2017
First decision: June 20, 2017
Article in press: August 25, 2017
P- Reviewer: Amiri M, Bang YJ, Caboclo JL, Komatsu S S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Ma YJ
Contributor Information
Ming-Chang Tsai, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung 40201, Taiwan; School of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan; Institute of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan.
Chi-Chih Wang, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung 40201, Taiwan; School of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan; Institute of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan.
Hsiang-Lin Lee, School of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan; Institute of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan; Department of Surgery, Chung Shan Medical University Hospital, Taichung 40201, Taiwan.
Cheng-Ming Peng, School of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan; Institute of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan; Department of Surgery, Chung Shan Medical University Hospital, Taichung 40201, Taiwan.
Tzu-Wei Yang, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung 40201, Taiwan; School of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan; Institute and Department of Biological Science and Technology, National Chiao Tung University, Hsinchu 30010, Taiwan.
Hsuan-Yi Chen, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung 40201, Taiwan; School of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan.
Wen-Wei Sung, School of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan; Institute of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan; Department of Urology, Chung Shan Medical University Hospital, Taichung 40201, Taiwan; Department of Medical Education, Chung Shan Medical University Hospital, Taichung 40201, Taiwan; Department of Medical Technology, Jen-Teh Junior College of Medicine, Nursing and Management, Miaoli 35664, Taiwan.
Chun-Che Lin, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung 40201, Taiwan; School of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan. cs1601@csmu.edu.tw; Institute of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan.
References
- 1.Pisani P, Parkin DM, Ferlay J. Estimates of the worldwide mortality from eighteen major cancers in 1985. Implications for prevention and projections of future burden. Int J Cancer. 1993;55:891–903. doi: 10.1002/ijc.2910550604. [DOI] [PubMed] [Google Scholar]
- 2.Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330–1344. doi: 10.1016/j.ejca.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 3.Zhu AL, Sonnenberg A. Is gastric cancer again rising? J Clin Gastroenterol. 2012;46:804–806. doi: 10.1097/MCG.0b013e3182604254. [DOI] [PubMed] [Google Scholar]
- 4.Coggon D, Barker DJ, Cole RB, Nelson M. Stomach cancer and food storage. J Natl Cancer Inst. 1989;81:1178–1182. doi: 10.1093/jnci/81.15.1178. [DOI] [PubMed] [Google Scholar]
- 5.La Vecchia C, Negri E, D’Avanzo B, Franceschi S. Electric refrigerator use and gastric cancer risk. Br J Cancer. 1990;62:136–137. doi: 10.1038/bjc.1990.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caruso ML, Fucci L. Histological identification of Helicobacter pylori in early and advanced gastric cancer. J Clin Gastroenterol. 1990;12:601–602. [PubMed] [Google Scholar]
- 7.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 9.Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst. 1968;40:43–68. [PubMed] [Google Scholar]
- 10.Haenszel W, Kurihara M, Segi M, Lee RK. Stomach cancer among Japanese in Hawaii. J Natl Cancer Inst. 1972;49:969–988. [PubMed] [Google Scholar]
- 11.Yong X, Tang B, Xiao YF, Xie R, Qin Y, Luo G, Hu CJ, Dong H, Yang SM. Helicobacter pylori upregulates Nanog and Oct4 via Wnt/β-catenin signaling pathway to promote cancer stem cell-like properties in human gastric cancer. Cancer Lett. 2016;374:292–303. doi: 10.1016/j.canlet.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Lee DS, Morrissey R, Aponte-Pieras JR, Rogers AB, Moss SF. Early or late antibiotic intervention prevents Helicobacter pylori-induced gastric cancer in a mouse model. Cancer Lett. 2015;359:345–351. doi: 10.1016/j.canlet.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 13.de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945–952. doi: 10.1053/j.gastro.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 14.Song H, Ekheden IG, Zheng Z, Ericsson J, Nyrén O, Ye W. Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ. 2015;351:h3867. doi: 10.1136/bmj.h3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li D, Bautista MC, Jiang SF, Daryani P, Brackett M, Armstrong MA, Hung YY, Postlethwaite D, Ladabaum U. Risks and Predictors of Gastric Adenocarcinoma in Patients with Gastric Intestinal Metaplasia and Dysplasia: A Population-Based Study. Am J Gastroenterol. 2016;111:1104–1113. doi: 10.1038/ajg.2016.188. [DOI] [PubMed] [Google Scholar]
- 16.Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666–673. doi: 10.1002/ijc.24290. [DOI] [PubMed] [Google Scholar]
- 17.Hamashima C, Ogoshi K, Narisawa R, Kishi T, Kato T, Fujita K, Sano M, Tsukioka S. Impact of endoscopic screening on mortality reduction from gastric cancer. World J Gastroenterol. 2015;21:2460–2466. doi: 10.3748/wjg.v21.i8.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen SL, Wang SC, Ho CJ, Kao YL, Hsieh TY, Chen WJ, Chen CJ, Wu PR, Ko JL, Lee H, et al. Prostate Cancer Mortality-To-Incidence Ratios Are Associated with Cancer Care Disparities in 35 Countries. Sci Rep. 2017;7:40003. doi: 10.1038/srep40003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordero-Morales A, Savitzky MJ, Stenning-Persivale K, Segura ER. Conceptual considerations and methodological recommendations for the use of the mortality-to-incidence ratio in time-lagged, ecological-level analysis for public health systems-oriented cancer research. Cancer. 2016;122:486–487. doi: 10.1002/cncr.29747. [DOI] [PubMed] [Google Scholar]
- 20.Sunkara V, Hébert JR. The application of the mortality-to-incidence ratio for the evaluation of cancer care disparities globally. Cancer. 2016;122:487–488. doi: 10.1002/cncr.29746. [DOI] [PubMed] [Google Scholar]
- 21.Sunkara V, Hébert JR. The colorectal cancer mortality-to-incidence ratio as an indicator of global cancer screening and care. Cancer. 2015;121:1563–1569. doi: 10.1002/cncr.29228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varghese C, Carlos MC, Shin HR. Cancer burden and control in the Western Pacific region: challenges and opportunities. Ann Glob Health. 2014;80:358–369. doi: 10.1016/j.aogh.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Seo JY, Jin EH, Jo HJ, Yoon H, Shin CM, Park YS, Kim N, Jung HC, Lee DH. Clinicopathologic and molecular features associated with patient age in gastric cancer. World J Gastroenterol. 2015;21:6905–6913. doi: 10.3748/wjg.v21.i22.6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.BERG HH. [Forms of stomach cancer with long survival rate after surgery and early diagnosis] Radiol Clin. 1954;23:1–4. [PubMed] [Google Scholar]
- 25.Lee CM, Choi IK, Kim JH, Park DW, Kim JS, Park SH. Is noncurative gastrectomy always a beneficial strategy for stage IV gastric cancer? Ann Surg Treat Res. 2017;92:23–27. doi: 10.4174/astr.2017.92.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiraishi K, Mimura K, Izawa S, Inoue A, Shiba S, Maruyama T, Watanabe M, Kawaguchi Y, Inoue M, Fujii H, et al. Lapatinib acts on gastric cancer through both antiproliferative function and augmentation of trastuzumab-mediated antibody-dependent cellular cytotoxicity. Gastric Cancer. 2013;16:571–580. doi: 10.1007/s10120-012-0219-5. [DOI] [PubMed] [Google Scholar]
- 27.Wang SC, Sung WW, Kao YL, Hsieh TY, Chen WJ, Chen SL, Chang HR. The gender difference and mortality-to-incidence ratio relate to health care disparities in bladder cancer: National estimates from 33 countries. Sci Rep. 2017;7:4360. doi: 10.1038/s41598-017-04083-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


