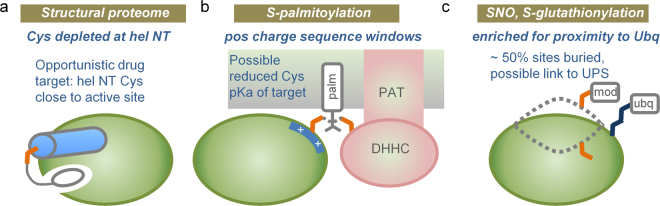
Figure 10.
Bioinformatics insights to cysteine reactivity. (a) Depletion of cysteine at α-helical amino-termini, relative to aspartic acid and serine, suggests that cysteine reactivity is modulated in evolution. Where Cys at a helical N-terminus does occur, and where this is adjacent to an enzyme active (or other modulatory) site, scope exists for targeting with covalent inhibitors. (b) S-palmitoylation sites in particular are enriched for a positively-charged sequence surround, which could lower the Cys pKa, leading to more favourable transfer from the DHHC domain of a PAT. (c) Most notably for sites of S-nitrosylation and S-glutathionylation, about half of modified sites are buried in native structure, and sites are enriched for proximity to lysine ubiquitinations. One possibility is that cysteine modification could be linked to UPS-mediated degradation.