Abstract
Cell membrane thyroid hormone (TH) transport can be facilitated by the monocarboxylate transporter 8 (MCT8), encoded by the solute carrier family 16 member 2 (SLC16A2) gene. Human mutations of the gene, SLC16A2, result in the X-linked-inherited psychomotor retardation and hypomyelination disorder, Allan-Herndon-Dudley syndrome (AHDS). We posited that abrogating MCT8-dependent TH transport limits oligodendrogenesis and myelination. We show that human oligodendrocytes (OL), derived from the NKX2.1-GFP human embryonic stem cell (hESC) reporter line, express MCT8. Moreover, treatment of these cultures with DITPA (an MCT8-independent TH analog), up-regulates OL differentiation transcription factors and myelin gene expression. DITPA promotes hESC-derived OL myelination of retinal ganglion axons in co-culture. Pharmacological and genetic blockade of MCT8 induces significant OL apoptosis, impairing myelination. DITPA treatment limits OL apoptosis mediated by SLC16A2 down-regulation primarily signaling through AKT phosphorylation, driving myelination. Our results highlight the potential role of MCT8 in TH transport for human OL development and may implicate DITPA as a promising treatment for developmentally-regulated myelination in AHDS.
Keywords: NKX2.1, Human embryonic stem cells, Monocarboxylate transporter 8, Allan-Herndon-Dudley syndrome, Thyroid hormone, Di-iodothyropropionic acid (DITPA), Oligodendrocytes
Graphical Abstract
Highlights
-
•
NKX2.1-based sorting enhances OL derivation from hESC
-
•
MCT8 is required for the survival of OL precursor cells
-
•
DITPA promotes OL differentiation and myelination
-
•
DITPA overrides SLC16A2 (MCT8) down-regulation to potentiate myelination
Thyroid hormone is vital for oligodendrocyte differentiation and myelination. Lee and colleagues show that MCT8 is an integral thyroid hormone transporter for oligodendrocytes derived from human embryonic stem cells. Knockdown of this transporter induces apoptosis of OLs, which could be prevented by the provision of DITPA.
1. Introduction
Thyroid hormones (THs) play a vital role during mammalian embryonic brain development. Identification of a number of TH transporters, across cell plasma membranes, has expanded our understanding of how bioactive THs can elicit cell-specific developmental events (for review see Kapoor et al., 2015, Lee and Petratos, 2016). Membrane-bound transporters are members of the solute carrier (SLC) superfamily of proteins, now known to consist of 395 distinctive proteins categorized into 52 families (for review see Lin et al., 2015). The monocarboxylate transporters (MCTs), encoded by the SLC16 gene family, consist of 14 members, of which MCT8 (encoded by SLC16A2) and MCT10 (encoded by SLC16A10) are two homologous 12 transmembrane helical proteins, responsible for TH transport (for review see Visser et al., 2008). Other important TH membrane transporters include the organic anionic transporter protein (OAT1P1C) and the L-type amino acid transporters (LAT1 and LAT2) encoded by the SLCO1C1, SLC7A5 and SLC7A6 genes respectively (Tamai et al., 2000; Pizzagalli et al., 2002; Hagenbuch and Meier, 2003; Friesema et al., 2001). These transporters seem to be of critical importance at the blood brain barrier (BBB) for T4 transport from the peripheral circulation into the CNS parenchyma via astrocytes (Boado et al., 1999; van der Deure et al., 2008), although the levels of OAT1P1C in humans is considerably lower than that present in the rodent (Roberts et al., 2008), which may indicate why neurological phenotypes are present only in Mct8/Oat1p1c double knock out mice (Mayerl et al., 2014). Recently the identification of Mct8 expression in a rodent oligodendroglial cell line (158N) has been reported but its significance has not been elucidated (Braun et al., 2011).
Of clinical importance, MCT8 has been identified as a high affinity TH cell membrane transporter, since the only substrates identified include tri-iodothyronine (T3) and its pro-hormone thyroxine (T4) (Friesema et al., 2003; Kinne et al., 2010). In humans, mutations at the SLC16A2 gene locus (encoding MCT8) cause the severe congenital X-linked psychomotor retardation, known as Allan-Herndon-Dudley syndrome (AHDS) (Friesema et al., 2004; Dumitrescu et al., 2004; Schwartz et al., 2005). Along with the increased serum levels of free-T3, developmentally delayed myelination shown by magnetic resonance imaging (MRI), is a common feature of this disorder (Armour et al., 2015; Vaurs-Barriere et al., 2009; Gika et al., 2010). Although myelination was reported in T2-weighted MRI from follow-up longitudinal studies of AHDS patients, their brain development is incomplete as neurological phenotypes persist (Armour et al., 2015; Gika et al., 2010; Vaurs-Barriere et al., 2009). However, myelin deficits have been reported in a recent post-mortem analysis of an 11-year-old AHDS boy which revealed prominent hypomyelination by myelin basic protein (MBP) immunostaining (Lopez-Espindola et al., 2014).
Despite TH-dependency during oligodendrocyte (OL) differentiation, cell entry of these hydrophobic hormones remains undefined within this cell lineage. Since Slc16a2 mutant mice display no overt neurological abnormality, having only a mild behavioral phenotype (Dumitrescu et al., 2006; Wirth et al., 2009), we utilized oligodendroglial precursor cells (OPCs) derived from human embryonic stem cells (hESCs) to identify the expression profiles and physiological role of MCT8 during OL development. Although several protocols exist that derive OPCs from hESCs (for review see Alsanie et al., 2013), the efficiency to develop homogeneous cultures varies, preventing robust molecular analyses of derived OPCs and mature OLs. Therefore, we developed a modified technique to obtain high yields of oligodendroglial cells to clarify the role of MCT8 during OL development.
TH analogs that do not require MCT8 have been suggested as a potential therapy to treat AHDS. For example, di-iodothyropropionic acid (DITPA) can normalize peripheral hyperthyroidism and reduce hypermetabolism in AHDS patients (Verge et al., 2012). However, the exact mechanism by which DITPA acts is largely unknown. Considering our findings of reduced OL viability upon inhibition of MCT8, in this study we posit that the provision of DITPA upon knockdown of SLC16A2 in hESC-derived OPCs may potentiate their proliferation and differentiation. Microarray analysis revealed up-regulation of OL-specific transcription factors upon DITPA administration to early OPCs. We tested the effect of DITPA upon OL development and found that it induced cell cycle exit, OPC differentiation and myelination in vitro. Importantly, DITPA administration rescued these cells from apoptosis mediated by SLC16A2 down-regulation and promoted their myelination of axons, likely due to downstream phosphorylation of AKT and ERK1. Collectively, these data suggest that MCT8 is a physiological TH transporter in OLs and that early intervention using DITPA holds therapeutic promise in enhancing myelination in AHDS.
2. Materials and Methods
2.1. hESC Culture
We used two distinct lines of hESC for this study, HES3 and NKX2.1-GFP reporter line derived from HES3 (Goulburn et al., 2011). hESC studies were approved by Monash University Human Research Ethics Committee.
2.2. Derivation of OPCs
OPCs were generated from NKX2.1-GFP reporter line using our protocol modified from (Chaerkady et al., 2011; Kerr et al., 2010). A detailed protocol is described in Supplemental Experimental Procedures and defined within the International (PCT) Patent Publication No. WO2016/101017A1 (Petratos et al., 2016).
2.3. Immunocytochemistry
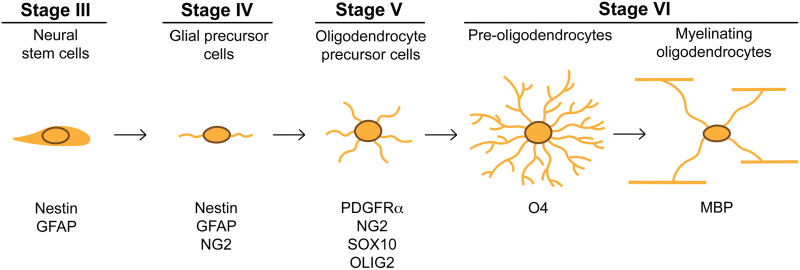
Preparations of cultures for immunolabeling are described in Supplemental Experimental Procedures. See Table 1 for definition of markers used to assess oligodendrocyte development.
Table 1.
Neural lineage markers utilized throughout this study.
| Name of the markers | Abbreviation | Expressed in | According to hESC differentiation (see Fig. 1) |
|---|---|---|---|
| Nestin | Nestin | Neural precursor cells | Stage IV onwards |
| Glial fibrillary acidic protein | GFAP | Neural precursor cells, Astrocytes | |
| Epcam | Epcam | Neuroepithelial cells | |
| β-III tubulin | β-III tubulin | Neurons | |
| Microtubule-associated protein 2 | MAP2 | Neurons | |
| Sex-determining region Y-box 10 | Sox10 | Oligodendrocyte precursor cells | Stage IV and V |
| Oligodendrocyte transcription factor 2 | Olig2 | Oligodendrocyte precursor cells | |
| Platelet-derived growth factor receptor alpha | PDGFRα | Glial precursor cells, Oligodendrocyte precursor cells |
|
| Chondroitin sulfate proteoglycan (CSPG4), Neural/glial antigen 2 |
NG2 | Glial precursor cells, Oligodendrocyte precursor cells |
|
| O4 | O4 | Pre-myelinating oligodendrocytes | Stage V and VI |
| Myelin basic protein | MBP | Mature oligodendrocytes | Stage VI |
 | |||
2.4. Flow Cytometry
Preparations of cells for flow cytometry are described in Supplemental Experimental Procedures. See Table 1 for definition of markers used to assess oligodendrocyte development.
2.5. Fluorescence-Activated Cell Sorting (FACS)
The rationale and detailed protocol for NKX2.1-based sorting is described in Supplemental Experimental Procedures. At the end of stage III of hESC differentiation, NKX2.1-GFP + cells were sorted using a BD Influx (BD Biosciences). The sorted cells; GFP − and GFP + cells were collected in stage IV medium with 10 μM Y27632 (Enzo) then further differentiated.
2.6. Microarray Analysis
H9 cell line-derived human (h)OPCs (Merck/Millipore) were differentiated for 4 weeks and the following treatments were administered to the cells for 48 h: Medium with 0.01% absolute ethanol (diluent control); 1 ng/mL DITPA (DITPA 1 ng/mL); 10 ng/mL DITPA (DITPA 10 ng/mL); or 100 ng/mL DITPA (DITPA 100 ng/mL). DITPA was diluted in absolute ethanol. 1 μg of collected mRNA from each population was hybridized to Human HT-12 v3.0 Gene Expression BeadChip (Illumina) according to the manufacturer's instruction (For detailed procedure see Supplemental Experimental Procedures).
2.7. Cell Cycle Analysis with BrdU
BrdU cell cycle analysis was performed according to the manufacturer's protocol (BD Biosciences). For a detailed protocol, see Supplemental Experimental Procedures.
2.8. Generation of Lentivirus Carrying SLC16A2-shRNA
psi-LVRU6MP vectors carrying either scrambled shRNA or 4 different shRNA sequences for SLC16A2 were generated by Genecopoeia, USA. These vectors have two different promoters, U6 promoter for shRNA and EF1α for the mCherry reporter, and a puromycin resistance for stable selection (8357 bp). We tested 4 different shRNA sequences for our NKX2.1-GFP hESC lines and the most efficient SLC16A2-shRNA sequence was selected for the generation of lentivirus. The most efficient target sequence for our cells is as follows: GCTTCGCGCCGTAGTCTTA. This specific sequence or scrambled shRNA sequence were packaged into a lentivirus (Genecopoeia).
2.9. Stable Knockdown of SLC16A2
NKX2.1-GFP sorted cells at the end of stage VI or V were transduced with psi-LVRU6MP vectors carrying either scrambled shRNA or shRNA sequences for SLC16A2 (Genecopoeia, USA) with multiplicity of infection (MOI) of 10 in appropriate stage-specific medium containing polybrene (5 μg/mL, Sigma-Aldrich). Efficiency of transduction was validated by analyzing mCherry + cells by flow cytometry and efficiency of knockdown was validated by analyzing the SLC16A2 transcript level by qRT-PCR 5 days post-transduction. An apoptosis assay was performed on cells either, treated with or without DITPA for 3 days after 72 h post-transduction, and then fixed. Cells were stained with monoclonal rat anti-mCherry (M11217, Life Technologies, 1:1000), polyclonal rabbit anti-cleaved caspase-3 (9661, Cell Signaling Technology, 1:400) and DAPI (Life Technologies) then analyzed by confocal microscopy (Nikon A1 Inverted using a × 20 water objective lens). Apoptotic OLs were defined as those mCherry-positive cells with cleaved caspase-3 + nuclei that were also condensed and fragmented as assessed by DAPI. The data were plotted as the number of cleaved caspase-3/mCherry + cells divided by total number of mCherry + cells. For the Western blotting performed to assess AKT and ERK1/2 signaling, see Supplemental Experimental Procedures.
2.10. Co-culture and Myelination Assays with Rat RGC and hESC-Derived OPCs
Retinae were dissected from P6 Sprague-Dawley rat pups (AMPREP Animal Ethics Committee #E-1602/2015/M). RGCs were purified according to the published immunopanning protocol (Watkins et al., 2008; Deliyanti and Wilkinson-Berka, 2015). Details of the RGC preparation are described in Supplemental Experimental Procedure. The RGC growth medium was changed every third day and cultures were maintained for 9 days. Pre-OLs from NKX2.1-GFP + cells at stage VI day 10, were FACS sorted according to O4 antibody labeling. O4 + sorted cells were then co-cultured with RGCs at a density of 20,000/well under 3 different conditions: T3 with 0.01% ethanol, 10 ng/mL DITPA, and 40 ng/mL T3 with DITPA in myelination medium (see Supplemental Experimental Procedure). The medium was changed every 3 days. At day 7, all co-cultures were fixed with 2% paraformaldehyde (PFA) for 10 min at room temperature. Cells were stained with a monoclonal rat anti-mCherry (Life Technologies, M11217, 1:1000); monoclonal mouse anti-NF-200 (Sigma-Aldrich, N0142, 1:200); and polyclonal rabbit anti-MBP (Millipore, AB980, 1:200). Subsequently cells were stained with appropriate Alexa Fluor-labeled secondary antibodies (Life technologies).
2.11. Quantification of Myelination in Culture
In RGC-OPC co-cultures, myelin segments were counted manually. 10 fields (× 20 objective) were randomly selected and captured from each coverslip with a confocal microscope (Nikon A1 inverted). For representative images, 10 z-stack images (0.25 μm intervals) were captured, analyzed and processed for 3D volume rendering by Imaris version 7.6.4. OLs were scored according to their morphology and defined as either “resting” (processes not touching axons); “contacting” (processes touching axons but not wrapping); and “ensheathing” (processes aligned with and wrapping axons). A myelination index (i.e. percentage of myelinated axons) was calculated by: the number of MBP + membrane segments that are ensheathing the NF200 + axons divided by the total number of NF200 + axons and multiplied by 100 (see Supplemental Experimental Procedure).
2.12. Statistical Analysis
Data are presented as mean ± SEM. Two-way ANOVA with Tukey's multiple comparison test determined statistical significance, unless otherwise stated. A P value of < 0.05 was considered as statistically significant. GraphPad Prism version 6.0c software was used for statistical analysis of the data.
3. Results
3.1. Patent
All data presented in this manuscript are defined within the International Patent Cooperation Treaty (PCT), International publication No. WO2016/101017A1 in the name of NeuOrphan Pty Ltd. Entitled: Improvements in oligodendroglial cell culturing methods and in methods for treating neurodegenerative disorders by using thyroid hormones or analogues (Petratos et al., 2016). NeuOrphan Pty Ltd. has commercial interests in the current technology for research and development purposes.
3.2. NKX2.1-GFP-based Sorting Enhances OPC Yield
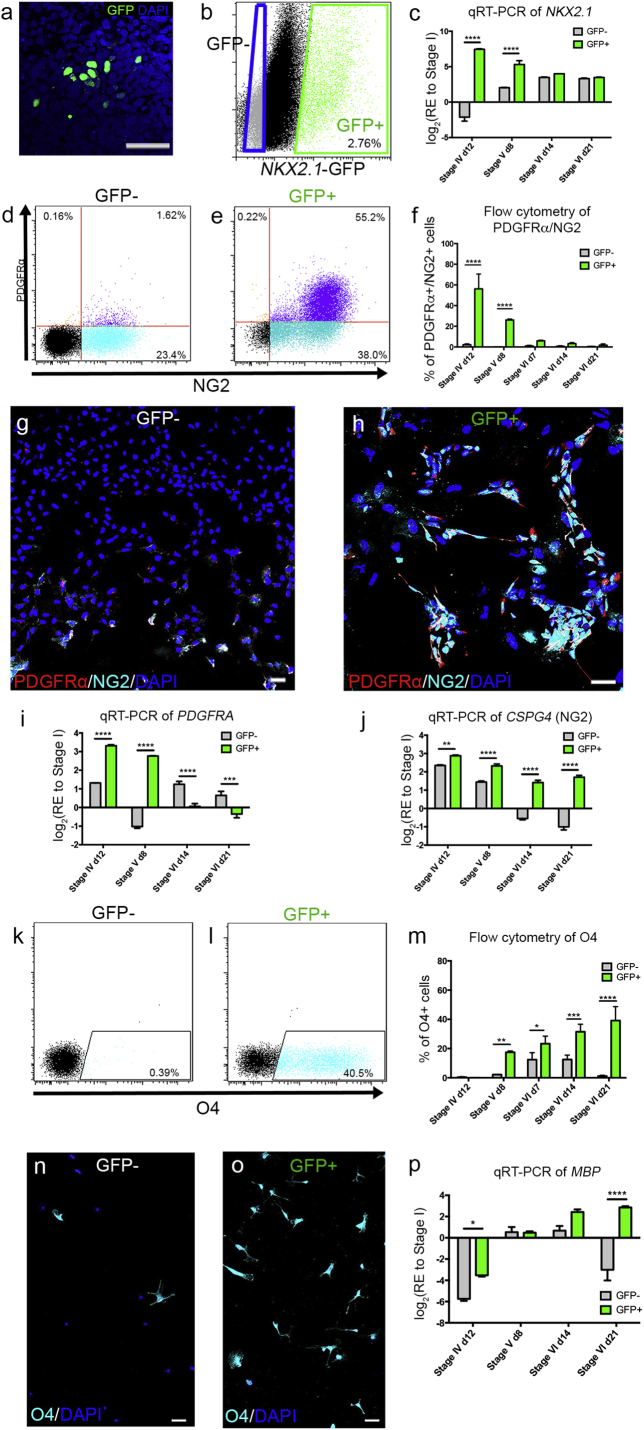
We utilized two hESC lines in this study; Hes3 and Hes3-derived NKX2.1-GFP reporter lines (Goulburn et al., 2011). The previous protocol for generating hESC-derived OPCs (Chaerkady et al., 2011) was modified with the addition of sonic hedgehog (shh) (Pringle et al., 1996) during the neural precursor stage of differentiation (Fig. 1) [see also the International (PCT) Patent Application No. PCT/AU2015/000770]. We successfully derived PDGFRα +/NG2 + OPCs, O4 + pre-OLs and MBP + pre-myelinating OLs (Fig. 1). By flow cytometry and immunocytochemistry, we identified the induction of PDGFRα +/NG2 + OPCs and O4 + pre-OLs at the end of stage IV and VI, respectively but at low yields. We then sorted NKX2.1 + cells at the peak timepoint of GFP expression during hESC differentiation. By live imaging and flow cytometry, we observed NKX2.1-GFP induction at day 8 of stage II (Fig. S1). During stage III, we identified that the maximal induction of NKX2.1-GFP occurred by day 5, served as a time-point for cell sorting (Fig. 2A,B). Therefore, both GFP + and GFP − cells were sorted by FACS at day 5 of stage III, then differentiated under the same culture conditions upon which their oligodendrogenic potential was addressed. Following sorting of the GFP + and GFP − populations of differentiating hESCs according to maximal induction of NKX2.1-GFP, qRT-PCR analysis of the NKX2.1 transcript identified significant up-regulation of this gene in GFP + populations at stages IV-V (Fig. 2C). First, the yield of PDGFRα +/NG2 + OPC derivation was analyzed. At the end of stage IV, the yield of OPC derivation was significantly higher in the GFP + (~ 55.2%) compared with GFP − (~ 1.62%) isolated cells (Fig. 2D,E). This trend was also evident at stage V day 6, although the percentage of PDGFRα +/NG2 + OPCs significantly decreased as the cells differentiated (Fig. 2F, Fig. S2A—B). Immunocytochemistry for PDGFRα and NG2 at stage IV day 12, supported the flow cytometry data (Fig. 2G,H). Furthermore, in line with these data we also showed significant up-regulation in PDGFRa from stage IV day 12, to stage V day 8, whereas the CSPG4 (encoding NG2) gene was significantly up-regulated early from stage IV onwards in GFP + compared with GFP − sorted cells (Fig. 2I,J).
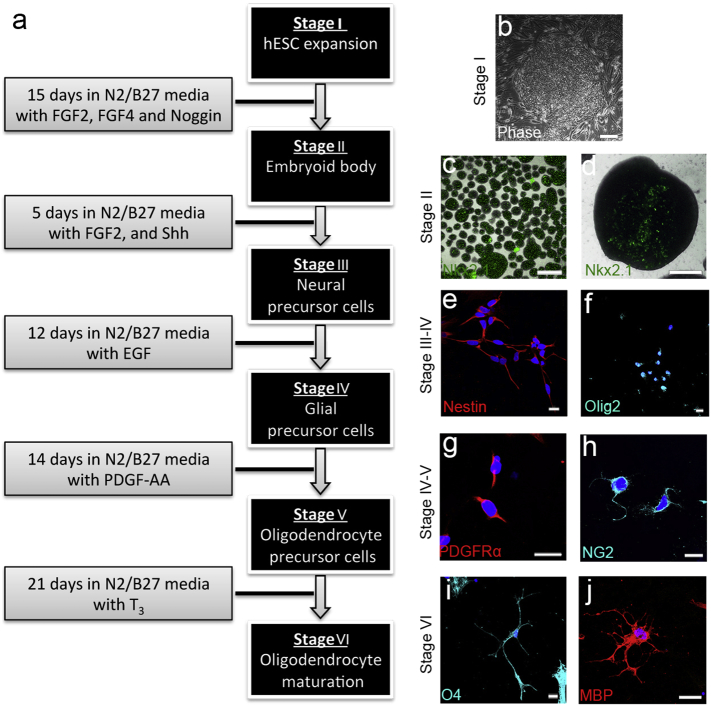
Fig. 1.
NKX2.1-GFP hESC reporter line can be directed toward an OPC fate.
(a) Directed differentiation of hESC into OPCs. (b) hESCs (stage I) were differentiated to neural embryoid bodies (EBs) (stage II) expressing (c, d) NKX2.1. (e) Nestin + neural precursor cells (stage III) were generated. (f) Olig2 + Glial precursor cells (stage IV) appeared under the influence of EGF. These were further differentiated into OPCs (stage V) expressing (g) PDGFRα and (h) NG2 through the addition of PDGF-AA. (i, j) T3 promoted terminal differentiation of OPCs into (i) O4 + pre-OLs (stage VI), and (j) MBP + pre-myelinating OLs. (e–j) Counterstaining was performed by DAPI. Scale bar = 100 μm for (b–d) and 20 μm for (e–j).
Fig. 2.
NKX2.1-GFP + sorted cells were differentiated toward an oligodendroglial lineage.
(a) hESC-derived NKX2.1-expressing cells were identified by immunolabeling for GFP followed by DAPI counterstaining. (b) GFP + cells were FACS-sorted from GFP-cells at stage III, day 5. (c) qRT-PCR analysis of NKX2.1 post-sorting throughout differentiation. (d–e) Flow cytometric analysis of PDGFRα +/NG2 + OPCs in (d) the GFP − and (e) GFP + populations at stage IV, day 12. (f) The proportion of PDGFRα +/NG2 + OPCs during differentiation. (g–h) Immunostaining for PDGFRα and NG2 on (g) GFP − and (h) GFP + sorted cells at stage V, counterstained with DAPI. (i–j) qRT-PCR analyses of i) PDGFRA and (j) CSPG4 throughout differentiation. (k-m) Flow cytometric analysis of O4 in (K) GFP − and (l) GFP + sorted populations at stage VI, day 21. (M) The proportion of O4 + immature OLs throughout differentiation. (n-o) Immunostaining for O4 and DAPI on (n) GFP − and (o) GFP + sorted populations. (p) qRT-PCR analysis of MBP throughout differentiation (RE; relative expression). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; (n = 3–4; mean ± SEM). Scale bar = 50 μm.
Next, we analyzed the yield of O4 + pre-OL derivation from stage IV to stage VI. We found that the percentage of O4 + pre-OLs was significantly higher in GFP + compared with GFP − sorted cells from stages V-VI (Fig. 2K–O). Immunocytochemistry analysis also demonstrated a greater derivation of O4 + pre-OLs at the end of stage VI (Fig. 2N,O). In support of these data, qRT-PCR analysis for the MBP gene showed a ~ 4-fold up-regulation in GFP + sorted cells when compared with GFP − isolated cells at stage VI day 21 (Fig. 2P). Further to these surface markers, we analyzed an essential transcription factor for oligodendrogenesis, Sox10 (Fig. S2C-F). By performing immunocytochemistry, we detemined that significantly more Sox10 + cells were generated from the isolated GFP + cells by stage IV (day 12; ~ 49%), and VI (day 21; ~ 82%), than GFP-negative cells (< 20% at either stage; Fig. S2E). qRT-PCR analysis supported these immunocytochemistry data, showing significantly up-regulated SOX10 gene expression levels in GFP + when compared with the GFP − sorted cells from stage IV day 12, to stage V day 8 (Fig. S2F).
3.3. MCT8 is Expressed on Oligodendroglial Lineage Cells
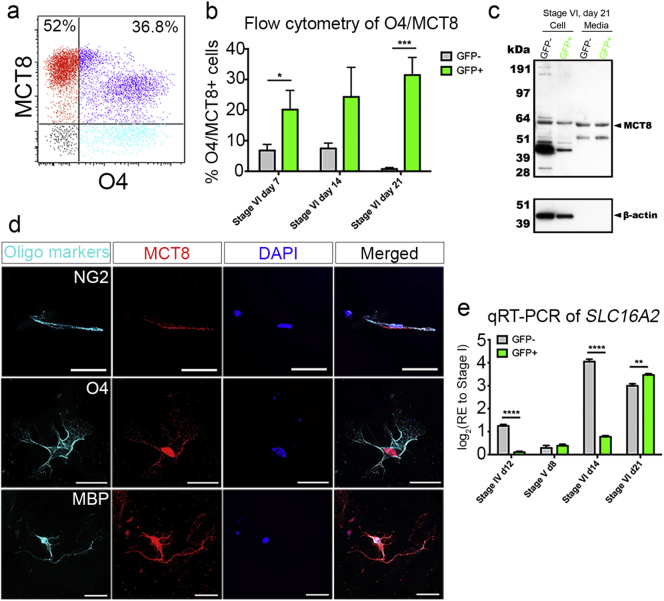
Having successfully generated human OPCs, immature OLs and OLs, we next aimed to determine which of these cell types expressed MCT8 was indeed co-expressed with various oligodendroglial lineage markers (Fig. 3). Firstly, we showed MCT8 expression on O4 + pre-OLs by flow cytometry during stage VI, where significant numbers of O4 + cells were labeled with MCT8 among the GFP + sorted cultures (Fig. 3A,B). Moreover, western blot analysis of cell lysates from stage VI, revealed a specific monomeric form of MCT8 (approximately 60 kDa) (Friesema et al., 2006) in both the GFP − and GFP + isolated cells (for commercial antibody validation assay see Fig. S4). We also detected this in the conditioned medium, indicating MCT8 may be released extracellularly (Fig. 3C), possibly enriched in oligodendroglial cell-derived exosomes (Fruhbeis et al., 2013). This possibility is currently under investigation. qRT-PCR analysis for SLC16A2 revealed increasing levels of this gene from the OPC stage (stage IV day 12) through to the pre-OL stage (stage VI day 21) in GFP + isolated cells (Fig. 3D). These results indicate that MCT8 is expressed in maturing oligodendroglial lineage cells.
Fig. 3.
MCT8 is expressed on immature and pre-myelinating OLs.
(a) Flow cytometric analysis showing expression of MCT8 in O4 + immature OLs derived from the GFP + sorted population at stage VI, day 21. (b) The proportion of O4 +/MCT8 + cells during differentiation (c) Prior to cell lysate protein analysis, commercial antibody validation as performed (see Fig. S4). Western blot of cell lysates and conditioned medium from the GFP − and GFP + sorted population at stage VI, day 21 with MCT8 and β-actin antibodies. (d) qRT-PCR analysis of SLC16A2 during differentiation. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; (n = 3; mean ± SEM). Scale bar = 50 μm.
3.4. DITPA Potentiates OL Development in Mixed Neural Cultures
To identify whether DITPA can promote oligodendrogenesis in the same manner as T3, we first utilized the human OPC differentiation kit (Merck-Millipore). Using this kit, we were able to derive ~ 30% of NG2 + OPCs at the end of differentiation (Week 4) (Fig. 4A). Further analysis revealed that there was a mixed neural population of Nestin + neural precursors, GFAP + astrocytes and β-III-tubulin + neurons at the end of differentiation (Week 4, data not shown), indicating these cells are at the early stage of OPC specification from multipotent neural precursors. From this time-point, we treated cells with 1 ng/mL and 10 ng/mL of DITPA for 48 h and performed microarray analysis. Cells grown in differentiation medium with 0.01% ethanol (dissolvent of DITPA) served as a negative control.
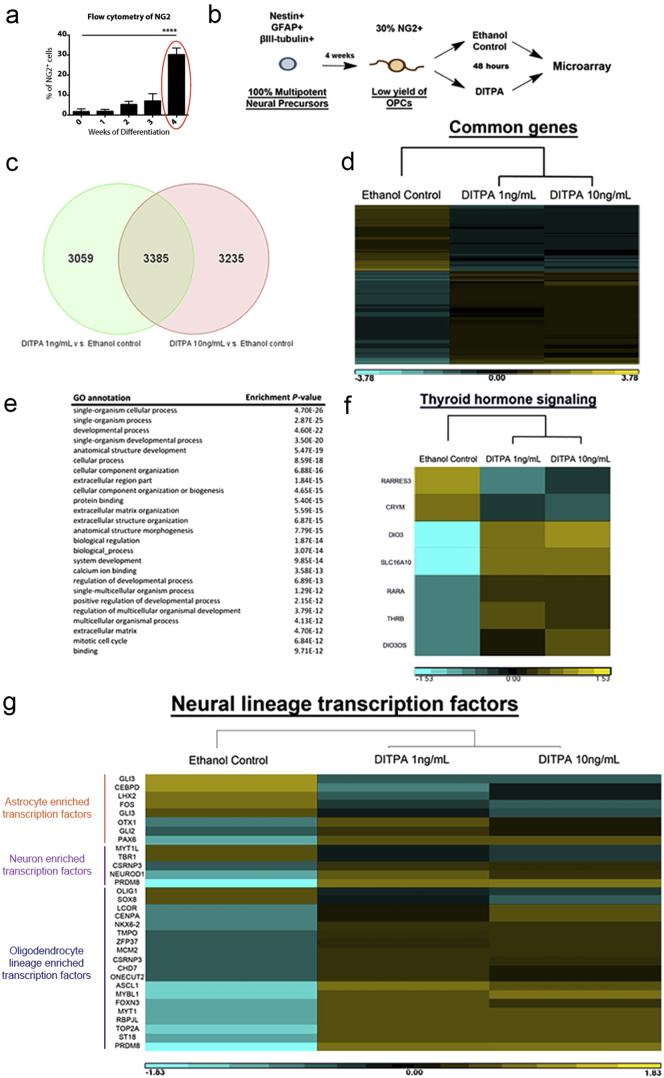
Fig. 4.
Transcriptome analysis for human mixed neural cell cultures differentiated toward OPC lineage with DITPA.
(a) The proportion of NG2-positive cells during differentiation of Merck-Millipore human mixed neural cell cultures. One-way ANOVA with Tukey's post-hoc test; ****P < 0.0001 (n = 3–4; mean ± SEM). At week 4, ~ 30% of NG2 + cells were derived and DITPA (10 ng/mL) was treated (circled in red). (b) DITPA treatment (10 ng/mL) regime upon human OPC specification from neural precursors. (c) Comparative gene expression profiles from different concentrations of DITPA (10 ng/mL) versus ethanol control (including T3 in differentiation medium) treatments. (d) Genome-wide transcriptional profile heatmap obtained for the different concentrations of DITPA (10 ng/mL) and ethanol control treatments. (e) Highly enriched GO terms of commonly expressed genes following the administration of DITPA at 1 ng/mL and 10 ng/mL versus ethanol control. (f and g) Heatmap of (f) genes related to TH signaling and (g) Neuronal, astroglial and oligodendroglial enriched transcription factors (Gene lists are from Najm et al., 2013) following the administration DITPA (10 ng/mL) and ethanol control.
Gene expression profiles of cells treated with either DITPA at 1 ng/mL or DITPA at 10 ng/mL when compared to control cells treated with Ethanol; showed that DITPA regulated 3385 genes (Fig. 4C), illustrated in the heatmap (Fig. 4D). Gene ontology (GO) analysis showed that genes related to developmental processes were regulated upon DITPA treatment (Fig. 4E). From this GO analysis, we selected the GO term ‘TH signaling’ and found a down-regulation of retinoic acid response element 3 (RARRES3) and intracellular TH-binding protein, μ-crystallin (CRYM). Moreover, there was an up-regulation of deiodinase 3 (DIO3), nuclear retinoic acid receptor alpha (RARA), nuclear TH receptor β (THRB) and DIO3 opposite strand (DIO3OS) (Fig. 4F). These results indicate that DITPA may not be transported by μ-crystallin in the cell since its cellular levels are required for appropriate TH signaling (Suzuki et al., 2007, Takeshige et al., 2014). Considering that DIO3, RARA and THRB were all upregulated in our GO analysis, it would suggest that TH signaling is maintained in these cell lines. However, one plausible hypothesis is that DITPA may bind to the retinoic acid receptor α and TH receptor β to transcribe OL-related genes (Baas et al., 2002, Baas et al., 2000, Laeng et al., 1994).
Importantly, we found up-regulation of an array of transcription factors (lists from Najm et al., 2013) enriched in oligodendroglial cells such as achaete-scute homolog 1 (ASCL1) and myelin transcription factor 1 (MYT1) with DITPA treatment whereas, minimal astrocyte and neuron enriched transcription factors were regulated upon DITPA treatment (Fig. 4G). Since T3 has been previously shown to promote cell cycle exit and OL differentiation (Barres et al., 1994), we further analyzed cell cycle associated gene pathways such as BMP, TGFβ, WNT, and Notch signaling (within the GO term). From this analysis, we found genes associated with these pathways to be regulated by DITPA (Fig. S5A-B). Furthermore, we found: (i) a significant up-regulation of the reprimo (RPRM) (~ 13 fold), a p53-dependent G2 arrest mediator (Laeng et al., 1994); (ii) up-regulation of metallothionein (MT1A) (~ 8 fold), a binding partner of p53 to modulate p53-dependent apoptosis (Ostrakhovitch et al., 2006); and (iii) the up-regulation of P53 (~ 2 fold) upon DITPA treatment (Fig. S5B). These results may indicate that DITPA promotes cell cycle exit and potentiates OL development at the early stage of differentiation.
3.5. DITPA Promotes Cell Cycle Exit to Potentiate OL Differentiation
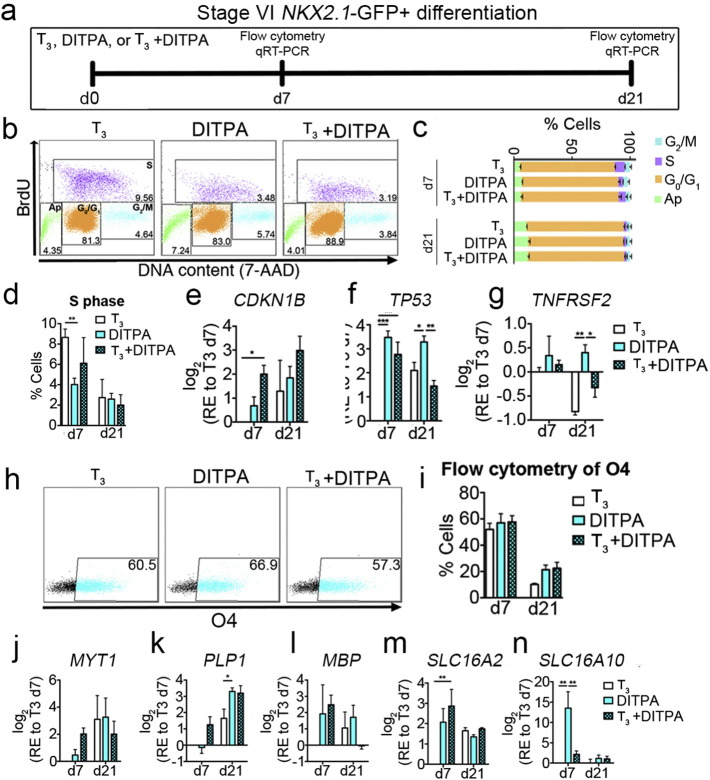
To examine the effect of DITPA on cell cycle exit, we treated stage VI NKX2.1-GFP + sorted cells with T3 alone (T3 control), DITPA alone (DITPA), or co-administration of T3 and DITPA (T3 + DITPA) for 21 days and analyzed BrdU incorporation by flow cytometry at day 7 and 21 (Fig. 5A). At day 7 and 21, the cells were pulsed with BrdU for 1 h and treated with DNase I for 45 min. At day 7, we found a significant reduction in the numbers of cells that were in S phase upon both the administration of DITPA in the cultures, compared with those treated with T3 alone. However, at day 21 we found no significant differences among all three groups (Fig. 5B–D). These data indicate that DITPA potentiates cell cycle exit faster than T3. However, the possibility exists that T3 administration may also be generating other proliferating neural lineage cells.
Fig. 5.
DITPA potentiates OPC cycle exit promoting differentiation in enriched OL cultures derived from NKX2.1-GFP + sorted hESCs.
(a) DITPA treatment (10 ng/mL) regime throughout Stage VI. Cells were treated with either T3 alone (T3, 50 ng/mL), DITPA alone (DITPA, 10 ng/mL) or T3 with DITPA (T3, 50 ng/mL + DITPA, 10 ng/mL) then these were analyzed on day 7 and 21 post-treatments by flow cytometry and qRT-PCR. (b) Flow cytometric dot plots upon BrdU incorporation at day 7 of treatments (Ap; apoptotic cells). (c) The percentage of cells at the different cell cycle stages at day 7 and 21 post-treatments. (d) The percentage of cells in S phase (BrdU-positive) for the different treatment groups are shown. (e–g) qRT-PCR analyses of cell cycle-associated genes; (e) CDKN1B, (f) TP53 and (g) TNFRSF2, 7 and 21 days post-treatments. (h) Flow cytometric analysis of O4 at day 7 post-treatments. (i) The percentage of O4 + cells analyzed by flow cytometry at day 7 and 21 post-treatments. (j–l) qRT-PCR analyses of myelin genes; (j) MYT1, (k) PLP1, (l) MBP, 7 and 21 days post-treatments. (m and n) qRT-PCR analyses of TH transporter genes; (m) SLC16A2 (MCT8), and (n) SLC16A10 (MCT10) 7 and 21 days post-treatments. *P < 0.05; **P < 0.01; ***P < 0.001; (n = 3; mean ± SEM).
TH-responsive cell cycle arrest associated genes such as CDKN1B (encodes for p27) (Fig. 5E) and TP53 (encodes for p53) (Fig. 5F) were both up-regulated by DITPA and T3 + DITPA when compared to the T3 treated cultures. As overexpression of p53 has been identified to potentiate apoptosis via death receptors (DRs) (Wosik et al., 2003), we analyzed one of the DRs, previously reported to promote oligodendrocytopathy, DR6 (Mi et al., 2011), and found almost negligible up-regulation of TNFRSF2 (encodes DR6) upon DITPA treatment. Hence, the up-regulation of TP53 by DITPA treatment is likely to promote cell cycle exit for the purpose of OL differentiation, not cell death (Fig. 5G). Along with these data, further gene expression analysis by qRT-PCR revealed that down-regulation of the essential genes for BMP signaling, BMP7, JNK signaling JUN, TGFβ signaling, TGFB3, and WNT signaling WNT5A upon DITPA treatment occurs, corroborating the hypothesis that DITPA can promote differentiation. Furthermore, up-regulation of the WNT antagonists such as FRZB, SFRP1, and SFRP2 were found upon DITPA treatment supporting this notion (Fig. S5C). These data indicate that DITPA potentiates oligodendroglial cell cycle exit, promoting differentiation at the late stage from OPCs toward O4 + pre-OLs.
In line with these data demonstrating modulation of genes regulating cell development, further qRT-PCR-based gene expression analysis revealed that master regulators of OL differentiation such as OLIG1, OLIG2 and SOX10 were up-regulated following DITPA treatment. Following DITPA treatment at day 7, the greatest increase in gene expression was demonstrated to occur in OLIG1 and OLIG2, far greater than that seen for T3. Moreover, important genes for OL development such as ASCL1, PDGFRA, and NKX6.2 were up-regulated following all treatment regimes. On the other hand, as cells mature, the immature OPC gene, CSPG4 was down-regulated in all three treatment groups (Fig. S5C). Moreover, myelin genes such as MYT1, PLP1, and MBP were up-regulated following the treatment of differentiating cultures with DITPA (Fig. 5J–N). In particular, at day 21 of DITPA treatment, we found substantial up-regulation of PLP1 than that documented for the cells treated with T3 alone (Fig. 5J–L). These results suggest that DITPA promotes OL differentiation and this is far greater than what T3 can facilitate.
Having established the OL derivation potential of DITPA, we then asked whether these genes associated with TH signaling were also modified. Firstly, we found a significant up-regulation of SLC16A2 and SLC16A10 upon DITPA treatment at day 7 when compared with T3. Our results showed a ~ 14-fold up-regulation of SLC16A10 following DITPA treatment (Fig. 5M and N). Further analysis revealed no significant differences in the gene expression for THRB, DIO2 and DIO3, between T3 and DITPA treatments, indicating that similar intracellular signaling is operative upon either T3 or DITPA stimulation of cells. Co-administration of T3 and DITPA showed significant up-regulation of THRB and DIO3 (Fig. S5C), which may indicate that cells are in a hyperthyroid state.
3.6. DITPA Promotes Myelination in Co-culture
Since the evidence argued that DITPA promotes OL differentiation, we next asked whether it also potentiates CNS myelination. For this, we set up a co-culture system comprising rat retinal ganglion cells (RGCs) and NKX2.1-GFP + sorted OPCs where, these cultures were treated with T3, DITPA, or T3 + DITPA. Seven days following co-culture, we were able to detect subsets of MBP + OLs that began initiating or wrapping axons with myelin membrane in all treatment groups, identified by MBP + segments around NF-200 + axons (Fig. 6B). Quantification revealed that more contacting or ensheathing MBP + OLs are observed in DITPA treated cultures than those observed following treatment with T3 alone (Fig. 6C). In line with this, the percentage of myelinated axons (number of myelinated axons divided by the total number of axons and multiplied by 100) was significantly enhanced with DITPA compared with T3 treatment alone (Fig. 6D) although, qualitatively the amount of myelin added per axon (MBP immunofluorescence intensity/axon) did not appear to be altered in the DITPA treated cultures (Fig. 6B). These data illustrate that DITPA drives myelination of axons from differentiating OLs expressing MCT8, in an expedited manner than can occur with T3 alone, under normal differentiation conditions.
Fig. 6.
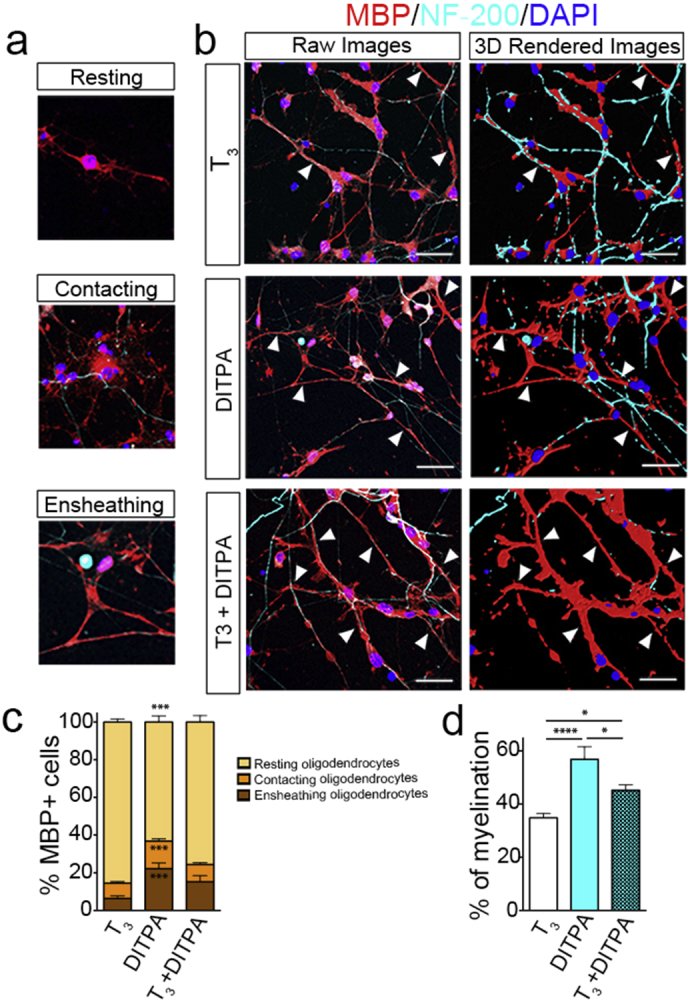
DITPA promotes the myelination of rat RGCs from NKX2.1-GFP + hESC derived OPCs.
(a–d) At day 10, stage VI, OPCs from NKX2.1-GFP + sorted cells were seeded on rat RGCs and maintained for 7 days with T3 (50 ng/mL), DITPA (10 ng/mL), or T3 (50 ng/mL) + DITPA (10 ng/mL) treatment, then immunostained with MBP, NF-200, and DAPI. MBP + OLs were scored for their morphology as (a) “Resting”, “Contacting”, or “Ensheathing”. (b) Representative deconvoluted z-stack captured images from the myelinating co-cultures treated with T3 (50 ng/mL), DITPA (10 ng/mL) or T3 (50 ng/mL) + DITPA (10 ng/mL). For better representation, these z-stack images were rendered into an artificial 3D image and shown below as raw images (arrowhead indicates regions of myelination, scale bars = 50 μm). (c) The percentage of MBP + OLs that are resting, contacting, and ensheathing from the different treatment groups. (d) The percentage of myelinated axons within the different treatment groups. One-way ANOVA with Tukey's post-hoc analysis; *P < 0.05; ***P < 0.001; ****P < 0.0001; (n = 9–10, mean ± SEM).
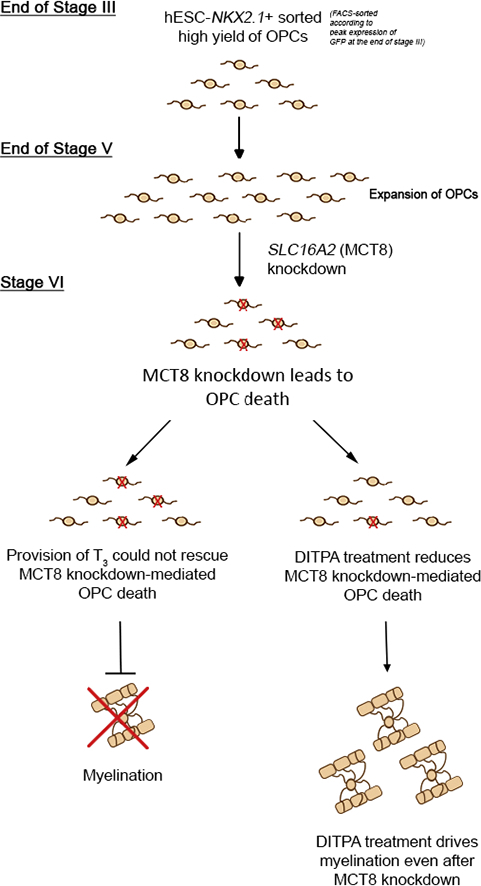
3.7. DITPA Limits OL Cell Death Caused by SLC16A2 Down-regulation and Promotes Myelination Under These Conditions
DITPA is suggested to cross the plasma membrane in a MCT8-independent manner. To confirm that DITPA can therefore overcome MCT8 deficiency in cells of the oligodendroglial lineage, we reduced MCT8 function pharmacologically and expression using a gene-knockdown approach. Firstly, we applied different concentrations (1 ng/mL, 10 ng/mL and 100 ng/mL) of Bosutinib to pre-OLs from NKX2.1-GFP + sorted cultures at stage VI, day 21 for 48 h. Bosutinib is a third generation tyrosine kinase inhibitor that was recently shown to non-competitively inhibit MCT8-dependent T3 and T4 uptake (Braun et al., 2012). By MTT cytotoxicity assay we showed increased viability in the hESC-derived oligodendroglial cell populations that were treated with DITPA (see Supplemental information Fig. S6).
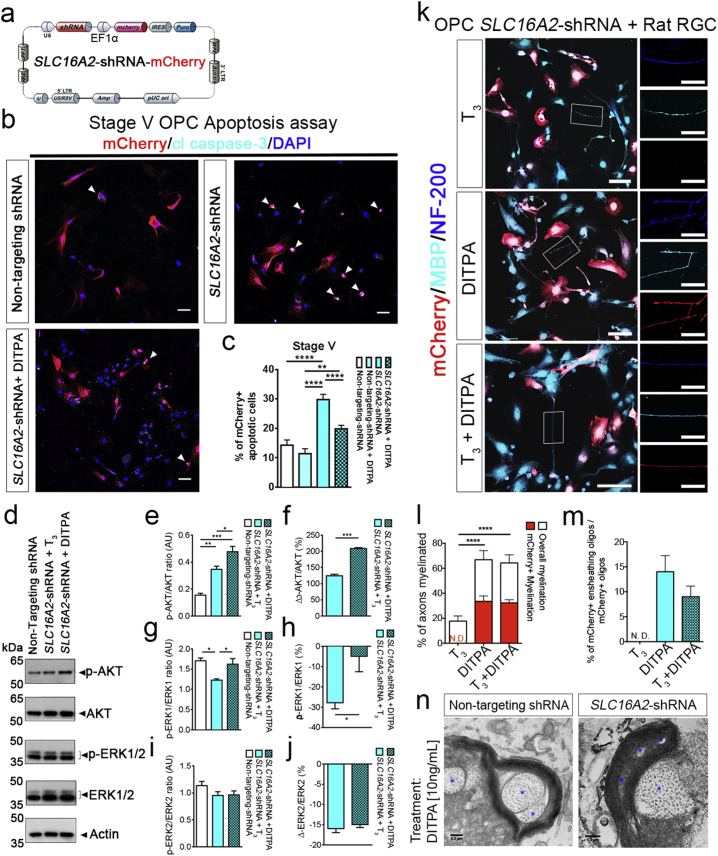
Moreover, we generated a lentivirus (LV) coding for SLC16A2- or scrambled shRNA (SLC16A2-shRNA – Fig. 7A). Pre-OLs from NKX2.1-GFP + sorted cultures at stage V, day 21 were transduced with either LV-SLC16A2-shRNA or LV-scrambled shRNA and harvested 5 days post-transduction, then these cells were used to examine gene expression by qRT-PCR and MCT8 protein expression by western blot. (Figs. 7, S7). We found knock-down efficiency of ~ 10-fold down-regulation of SLC16A2 by qRT-PCR and a substantial reduction in expression levels by western blot when compared with LV incorporating non-targeting shRNA (Fig. S7B).
Fig. 7.
DITPA rescues oligodendroglial cell death mediated by SLC16A2 knock down, retaining its myelination capability.
(a) Lentivirus (LV) was generated to carry the SLC16A2-shRNA with a mCherry reporter (b) Immunostaining for mCherry and cleaved caspase-3 (cl caspase-3) on NKX2.1-GFP + sorted OPCs at stage V, day 12 (3 days post-transduction with non-targeting, SLC16A2-shRNA, or SLC16A2-shRNA + DITPA (10 ng/mL)) counterstained with DAPI. (b and c) The proportion of mCherry +/cl caspase-3 + apoptotic cells was increased upon SLC16A2-shRNA transduction. DITPA treatment (10 ng/mL) reduced the SLC16A2-shRNA-mediated apoptosis. Scale bar = 50 μm. (d–j) Western blot analysis of lysates from OPCs transduced either with non-targeting-shRNA or SLC16A2-shRNA assayed for p-AKT and p-ERK1/2. (e–f) Densitometric quantification of p-AKT/total AKT, (G-H) p-ERK1/total ERK1, and (i–j) p-ERK2/total ERK2. (k) 3 days post-transduction with SLC16A2-shRNA, OPCs from NKX2.1-GFP + sorted cells were seeded on rat RGCs and were maintained for 7 days with T3 (50 ng/mL), DITPA (10 ng/mL), or T3 (50 ng/mL) + DITPA (10 ng/mL) treatment, then immunostained with mCherry, MBP and NF-200 Scale bar = 100 μm. Magnified images of single axons are shown on the right hand side of each image Scale bar = 20 μm. (l) The percentage of overall myelination and mCherry + myelination and (m) the percentages of ensheathing mCherry +/MBP + OLs among the different treatment groups. (n) Representative electron microscopic images of rat RGCs and OPCs co-cultures transduced with non-targeting, or, SLC16A2-shRNA upon DITPA treatment (10 ng/mL), showing compact myelin. Asterisks indicate axon fibers. Scale bar = 0.2 μm. One-way ANOVA with Tukey's post-hoc analysis; **P < 0.01; ****P < 0.0001; N.D.: not detected; (n = 9–10, mean ± SEM).
Having demonstrated that these cells had significantly reduced MCT8 expression (Fig. S6), we proceeded to examine the effect of MCT8 knockdown on the level of apoptosis in these cultures, and determined that after 5 days of LV transduction, significantly more mCherry-labeled cells were positive for cleaved caspase 3 in the SLC16A2-shRNA treated cultures relative to scrambled controls. The effect of MCT8 knockdown was overcome by the administration of DITPA from 3 days post-LV-transduction (Fig. 7B,C), indicating DITPA prevents MCT8 deprivation-induced cell death.
We followed up this line of investigation by identifying prominent molecular signaling events that maybe activated to potentiate DITPA-mediated OL survival against SLC16A2 knockdown. THs are known to regulate AKT and ERK1/2 signaling and these constitute downstream effectors of oligodendrocyte survival (Flores et al., 2000; Zaka et al., 2005). We analyzed the effect of DITPA on AKT and ERK1/2 signaling upon SLC16A2 knockdown (Fig. 7D-J). We found a significant elevation in the levels of AKT phosphorylation (p-AKT) among SLC16A2-shRNA transduced OPCs following DITPA treatment when compared with transduced cells treated with T3 alone or non-transduced OPCs. Furthermore, we observed a significant reduction in the levels of p-ERK1 upon SLC16A2 knockdown in the presence of T3. However, this survival effector phosphoprotein was normalized to basal levels (non-transduced control) upon DITPA treatment of transduced cells (Fig. 7D–J). These results suggest that DITPA may prevent the death of OPCs following SLC16A2 knockdown through the activation of AKT and ERK1 signaling.
Having demonstrated that DITPA can promote the survival of OPCs following acute downregulation of SLC16A2, we then set up the same co-culture experiment whereby rat RGCs were cultured with SLC16A2 down-regulated OLs (post-LV-transduction) from NKX2.1-GFP + sorted cells at stage V day 14 (Fig. 7K–N). We treated the cultures with T3, DITPA or, T3 + DITPA, on the day of co-culture and these were maintained for 7 days. mCherry +/MBP + myelinating OLs and mCherry +/MBP + myelin segments were found only in DITPA or T3 + DITPA treated cultures (Fig. 7L–M). Electron microscopic imaging of the co-cultures demonstrated ultrastructural organization of myelin surrounding RGC axons in both control (non-targeting shRNA) and SLC16A2-shRNA transduced cultures with DITPA treatment but not in SLC16A2-transduced cultures with T3 treatment (Fig. 7N). These data indicate that DITPA can promote the differentiation of OLs and moreover can potentiate the myelination of axons, even under the condition of acute MCT8 knockdown.
4. Discussion
Critical stages of the perinatal period govern brain development, where axons organized in fascicles are myelinated, thereby establishing time-dependent cognitive and motor functions. Hence, it is not surprising that the development of the fetal thyroid gland and circulating levels of THs are indeed elevated by the late gestational age, with maternal contributions delivered through the placenta (for review, see Moog et al., 2017, Bernal, 2007). Similarly, OL development coincides with circulating and CNS-specific fetal TH (Bernal, 2007). Despite the co-dependency of circulating and intracellular TH for brain development, oligodendrogenesis and myelination, the fact remains that both T3 and T4 need to enter developing neural cells to exert their genomic and non-genomic effects. In this study, we show that OL development can be intrinsically controlled by the function of the TH membrane transporter, MCT8. We identified that specific MCT8-deficiency in human OPCs can promote their cell death and that addition of the TH analog, DITPA can bypass such a deficiency to salvage OPCs and still promote their maturity toward myelinating OLs.
4.1. DITPA Modulates Gene Expression toward OPC Differentiation
Our data argue that down-regulation of oligodendroglial differentiation repressor genes of the Wnt-Notch signaling pathways can be achieved upon the administration of DITPA to hESC-derived OPCs. This raises the tantalizing hypothesis that DITPA can lift the repression imposed on myelin gene expression during OL development (Chew et al., 2011). Supporting this contention, we identified the up-regulations of WNT antagonists FRZB, SFRP1, and SFRP2 with DITPA treatment, again underpinning how DITPA can promote OL differentiation. However, recent evidence strongly suggests that in gastrointestinal tumors, the increased β-catenin-Tcf4 levels not only correlate with reduced TRα1 transcriptional activity on its target genes but are also likely responsible for the shift of TRα1 binding on Wnt targets (Sirakov et al., 2012) demonstrating a regulatory role for TH nuclear signaling in the cell cycle, all-be-it in a tumor cell line. Whether there is a causative effect of TH on Wnt or Notch pathways in neural cell differentiation including the derivation of mature OLs, has yet to be proven.
Along with the evidence supporting the action of TH directly on OL differentiation (Barres et al., 1994), T3 may affect OPC proliferation dependent on the cells' specific stage of development (Baas et al., 1997). OPCs have been shown to exhibit a limited number of divisions before terminal differentiation, with TH and retinoic acid acting as external signals that influence this timing (Ahlgren et al., 1997). In particular, downstream signaling through TRα1 following TH stimulation may be important in OPC differentiation, since Trα1−/− mice isolated OPCs continue to divide even in the presence of TH (Billon et al., 2002). Our data show that the effect of T3 on developing OLs derived from hESCs, is primarily seen from Stages V-VI, when the pre-OL marker O4 is expressed, coinciding with robust expression of the T3 membrane transporter MCT8. We showed that these O4 + OPCs consisted of a higher proportion of cells in S-phase, suggesting that T3 stimulation potentiates proliferation at this stage of development.
4.2. DITPA Promotes the Viability of Oligodendrocytes
Our data demonstrated that the down-regulation of, or functional blockade of MCT8 promotes cell death of OPCs, identifying the cell membrane-transport of T3 as integral to the expansion or depletion of OL populations. Intriguingly, we identified that the administration of DITPA to hESC-derived OPCs and other neural cell populations promoted the up-regulation of THRB and DIO3 suggesting that bypassing MCT8 can still potentiate TH genomic signaling in the context of OL maturation and myelin gene expression, similar to that observed by other thyromimetics (Baxi et al., 2014). Moreover, the downstream activation of the AKT and ERK1 non-genomic pathways were identified as prominent downstream effectors of DITPA promoting OPC viability.
4.3. DITPA Potentiates Oligodendrocyte Differentiation and Myelination in the Absence of MCT8
Despite the developmental dependency of OPCs on T3, no evidence exists for MCT8 during oligodendrogenesis and myelination. In mice, MCT8 facilitates the entry of TH into the brain parenchyma across the blood brain–barrier (BBB) (Ceballos et al., 2009) and, at a cellular level, the entry of TH into neurons in a region-specific manner (Trajkovic et al., 2007; Wirth et al., 2009). The importance of MCT8 for neurodevelopment was unequivocally demonstrated in patients genetically identified as AHDS (Vaurs-Barriere et al., 2009; Biebermann et al., 2005; Visser, 2013). These infants are usually clinically identified at the 6-month postnatal time point by virtue of their poor head control, a consequence of their general hypotonia. The hypotonia persists during development throughout the trunk and the peripheral hypotonia converts into dystonia and spasticity. These clinical manifestations are matched with a classically hypomyelinated brain profile which never reaches normal myelination throughout the individual's lifetime (Armour et al., 2015; Vaurs-Barriere et al., 2009). Currently, a clinical trial is underway using the TH analog, Triac (https://clinicaltrials.gov/ct2/show/NCT02060474) (Visser, 2014), and a previous off-label trial using DITPA for AHDS (Verge et al., 2012). There exists evidence that these MCT8-independent TH analogs can potentiate the resolution of peripheral hyperthyroid symptoms but their effect on human CNS cell development and importantly, clinical evidence of specific cognitive improvements, are still lacking (Verge et al., 2012; Kersseboom et al., 2014; Groeneweg et al., 2017; Ferrara et al., 2015; Ferrara et al., 2014). Our findings argue that the availability of T3 to human OPCs specifically can be restricted by a lack of functional MCT8 and can potentiate OL dystrophy.
An important implication for the effect of limited MCT8 function on myelin formation was established in a developmental zebrafish model where the ablation of the Slc16a2 gene rendered a OL maturation and myelin defect with associated locomotor and behavioral deficits (Zada et al., 2014). Indeed these resemble the neurological outcomes observed in AHDS patients under T2-weighted MRI (Armour et al., 2015) or following neuropathological assessment (Charzewska et al., 2016). It therefore appears that the zebrafish model may mimic the human TH regulation more closely than that exhibited in the mouse. This is further ratified by the data derived from the Slc16a2−/− mouse model that does not manifest the neurological phenotype that is exhibited in AHDS patients (Trajkovic et al., 2007). Moreover, it has since been identified that in the mouse CNS there is compensation for the loss of MCT8 through the upregulation of OATP1C1, an organic anion transporting polypeptide. The OATP1C1 transmembrane protein is capable of transporting T4 across the BBB, with compensatory increase in the astrocytic deiodinase 2 occurring in the Slc16a2−/− mouse model, converting T4 to T3 (Trajkovic et al., 2007). This was confirmed recently in a Slc16a2 and Oatp1c1 double knockout which exhibited the neurological deficits characteristic of AHDS, with reduced T3 and T4 uptake within the CNS and deiodinase activity with classical myelin delay (Mayerl et al., 2014). Our data support the effects seen in the zebrafish experimental paradigm since our hESC-derived OPCs had limited differentiation and myelinogenic potential following the acute LV-mediated shRNA knockdown of the SLC16A2 gene. Of greatest importance was the finding that DITPA treatment of the Slc16a2−/− zebrafish restored myelin deficiencies and locomotor behavioral outcomes (Zada et al., 2014). These data corroborate our findings that DITPA can potentiate OL differentiation and myelination in the absence of MCT8. The potential therapeutic window for use of DITPA in AHDS may relate to the heightened stages of oligodendrocyte maturation and myelination during the perinatal period (Groeneweg et al., 2017). In fact, it has been suggested that DITPA administration should be started in the mother in the last trimester if the MCT8 mutation is severe since transplacental passage of DITPA has been achieved in pregnant Mct8−/− dams, modulating neurodevelopmental genes within the cerebral cortex of their pups. If early detection of SLC16A2 mutations can be achieved within pregnant mothers in high-risk groups by genetic screening, then DITPA may be a viable option for parents carrying AHDS infants to term.
5. Conclusions
The current study has uncovered the biological outcomes of the DITPA when administered to differentiating OPCs. These cell-specific effects were the direct result of facilitated transcriptional regulation of OL differentiation and eventual myelination of CNS axons that could not be matched by T3 administration alone. The most profound pharmacological property of DITPA was that it was capable of salvaging OPCs deficient in MCT8 with a unique capacity to continue their differentiation toward myelination. These findings provide proof-of-principle data for the treatment of severe inherited neurodevelopmental disorders where TH metabolism is dysfunctional, such as the well-established CNS hypothyroid state occurring in AHDS afflicted children.
Funding Sources
JYL supported by Multiple Sclerosis Research Australia (MSRA) (Grant #12-060) postgraduate scholarship and Trish MS Foundation. SP supported by National Multiple Sclerosis Society grant #PP2232 and NeuOrphan P/L commercial agreement.
Authors' Contributions
JYL and SP: Conception and design, collection and/or assembly of data, data analysis and interpretation, and manuscript writing; MJK: Collection of data, manuscript writing; FR: Data analysis and interpretation; MFA, DD, JWB, AE and ES: Provision of study material.
Conflict of Interest
The authors declare that partial funding for the role of DITPA as a therapeutic molecule was through a commercial service agreement between Monash University and NeuOrphan P/L. No non-financial conflicts of interest exist for any of the authors.
Acknowledgments
Acknowledgements
We acknowledge the assistance of Ms. Pei Mun Aui and Ms. Kylie Magee for their technical and administrative support during the completion of this project.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.10.016.
Appendix A. Supplementary data
Supplementary material
References
- Ahlgren S.C., Wallace H., Bishop J., Neophytou C., Raff M.C. Effects of thyroid hormone on embryonic oligodendrocyte precursor cell development in vivo and in vitro. Mol. Cell. Neurosci. 1997;9:420–432. doi: 10.1006/mcne.1997.0631. [DOI] [PubMed] [Google Scholar]
- Alsanie W.F., Niclis J.C., Petratos S. Human embryonic stem cell-derived oligodendrocytes: protocols and perspectives. Stem Cells Dev. 2013;22:2459–2476. doi: 10.1089/scd.2012.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour C.M., Kersseboom S., Yoon G., Visser T.J. Further insights into the Allan-Herndon-Dudley Syndrome: clinical and functional characterization of a novel MCT8 mutation. PLoS One. 2015;10:e0139343. doi: 10.1371/journal.pone.0139343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas D., Bourbeau D., Sarlieve L.L., Ittel M.E., Dussault J.H., Puymirat J. Oligodendrocyte maturation and progenitor cell proliferation are independently regulated by thyroid hormone. Glia. 1997;19:324–332. doi: 10.1002/(sici)1098-1136(199704)19:4<324::aid-glia5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Baas D., Legrand C., Samarut J., Flamant F. Persistence of oligodendrocyte precursor cells and altered myelination in optic nerve associated to retina degeneration in mice devoid of all thyroid hormone receptors. Proc. Natl. Acad. Sci. U. S. A. 2002;99:2907–2911. doi: 10.1073/pnas.052482299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas D., Prufer K., Ittel M.E., Kuchler-Bopp S., Labourdette G., Sarlieve L.L., Brachet P. Rat oligodendrocytes express the vitamin D(3) receptor and respond to 1,25-dihydroxyvitamin D(3) Glia. 2000;31:59–68. doi: 10.1002/(sici)1098-1136(200007)31:1<59::aid-glia60>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Barres B.A., Lazar M.A., Raff M.C. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- Baxi E.G., Schott J.T., Fairchild A.N., Kirby L.A., Karani R., Uapinyoying P., Pardo-Villamizar C., Rothstein J.R., Bergles D.E., Calabresi P.A. A selective thyroid hormone beta receptor agonist enhances human and rodent oligodendrocyte differentiation. Glia. 2014;62:1513–1529. doi: 10.1002/glia.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 2007;3:249–259. doi: 10.1038/ncpendmet0424. [DOI] [PubMed] [Google Scholar]
- Biebermann H., Ambrugger P., Tarnow P., Von Moers A., Schweizer U., Grueters A. Extended clinical phenotype, endocrine investigations and functional studies of a loss-of-function mutation A150V in the thyroid hormone specific transporter MCT8. Eur. J. Endocrinol. 2005;153:359–366. doi: 10.1530/eje.1.01980. [DOI] [PubMed] [Google Scholar]
- Billon N., Jolicoeur C., Ying Q.L., Smith A., Raff M. Normal timing of oligodendrocyte development from genetically engineered, lineage-selectable mouse ES cells. J. Cell Sci. 2002;115:3657–3665. doi: 10.1242/jcs.00049. [DOI] [PubMed] [Google Scholar]
- Boado R.J., Li J.Y., Nagaya M., Zhang C., Pardridge W.M. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12079–12084. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun D., Kim T.D., Le Coutre P., Kohrle J., Hershman J.M., Schweizer U. Tyrosine kinase inhibitors noncompetitively inhibit MCT8-mediated iodothyronine transport. J. Clin. Endocrinol. Metab. 2012;97:E100–5. doi: 10.1210/jc.2011-1837. [DOI] [PubMed] [Google Scholar]
- Braun D., Kinne A., Brauer A.U., Sapin R., Klein M.O., Kohrle J., Wirth E.K., Schweizer U. Developmental and cell type-specific expression of thyroid hormone transporters in the mouse brain and in primary brain cells. Glia. 2011;59:463–471. doi: 10.1002/glia.21116. [DOI] [PubMed] [Google Scholar]
- Ceballos A., Belinchon M.M., Sanchez-Mendoza E., Grijota-Martinez C., Dumitrescu A.M., Refetoff S., Morte B., Bernal J. Importance of monocarboxylate transporter 8 for the blood-brain barrier-dependent availability of 3,5,3′-triiodo-l-thyronine. Endocrinology. 2009;150:2491–2496. doi: 10.1210/en.2008-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaerkady R., Letzen B., Renuse S., Sahasrabuddhe N.A., Kumar P., All A.H., Thakor N.V., Delanghe B., Gearhart J.D., Pandey A., Kerr C.L. Quantitative temporal proteomic analysis of human embryonic stem cell differentiation into oligodendrocyte progenitor cells. Proteomics. 2011;11:4007–4020. doi: 10.1002/pmic.201100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charzewska A., Wierzba J., Izycka-Swieszewska E., Bekiesinska-Figatowska M., Jurek M., Gintowt A., Klosowska A., Bal J., Hoffman-Zacharska D. Hypomyelinating leukodystrophies - a molecular insight into the white matter pathology. Clin. Genet. 2016;90:293–304. doi: 10.1111/cge.12811. [DOI] [PubMed] [Google Scholar]
- Chew L.J., Shen W., Ming X., Senatorov V.V., Jr., Chen H.L., Cheng Y., Hong E., Knoblach S., Gallo V. SRY-box containing gene 17 regulates the Wnt/beta-catenin signaling pathway in oligodendrocyte progenitor cells. J. Neurosci. 2011;31:13921–13935. doi: 10.1523/JNEUROSCI.3343-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliyanti D., Wilkinson-Berka J.L. Inhibition of NOX1/4 with GKT137831: a potential novel treatment to attenuate neuroglial cell inflammation in the retina. J. Neuroinflammation. 2015;12:136. doi: 10.1186/s12974-015-0363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Deure W.M., Hansen P.S., Peeters R.P., Kyvik K.O., Friesema E.C., Hegedus L., Visser T.J. Thyroid hormone transport and metabolism by organic anion transporter 1C1 and consequences of genetic variation. Endocrinology. 2008;149:5307–5314. doi: 10.1210/en.2008-0430. [DOI] [PubMed] [Google Scholar]
- Dumitrescu A.M., Liao X.H., Best T.B., Brockmann K., Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am. J. Hum. Genet. 2004;74:168–175. doi: 10.1086/380999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu A.M., Liao X.H., Weiss R.E., Millen K., Refetoff S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology. 2006;147:4036–4043. doi: 10.1210/en.2006-0390. [DOI] [PubMed] [Google Scholar]
- Ferrara A.M., Liao X.H., Gil-Ibanez P., Bernal J., Weiss R.E., Dumitrescu A.M., Refetoff S. Placenta passage of the thyroid hormone analog DITPA to male wild-type and Mct8-deficient mice. Endocrinology. 2014;155:4088–4093. doi: 10.1210/en.2014-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara A.M., Liao X.H., Ye H., Weiss R.E., Dumitrescu A.M., Refetoff S. The thyroid hormone analog DITPA ameliorates metabolic parameters of male mice with Mct8 deficiency. Endocrinology. 2015;156:3889–3894. doi: 10.1210/en.2015-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A.I., Mallon B.S., Matsui T., Ogawa W., Rosenzweig A., Okamoto T., Macklin W.B. Akt-mediated survival of oligodendrocytes induced by neuregulins. J. Neurosci. 2000;20:7622–7630. doi: 10.1523/JNEUROSCI.20-20-07622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesema E.C., Docter R., Moerings E.P., Verrey F., Krenning E.P., Hennemann G., Visser T.J. Thyroid hormone transport by the heterodimeric human system L amino acid transporter. Endocrinology. 2001;142:4339–4348. doi: 10.1210/endo.142.10.8418. [DOI] [PubMed] [Google Scholar]
- Friesema E.C., Ganguly S., Abdalla A., Manning Fox J.E., Halestrap A.P., Visser T.J. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J. Biol. Chem. 2003;278:40128–40135. doi: 10.1074/jbc.M300909200. [DOI] [PubMed] [Google Scholar]
- Friesema E.C., Grueters A., Biebermann H., Krude H., Von Moers A., Reeser M., Barrett T.G., Mancilla E.E., Svensson J., Kester M.H., Kuiper G.G., Balkassmi S., Uitterlinden A.G., Koehrle J., Rodien P., Halestrap A.P., Visser T.J. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364:1435–1437. doi: 10.1016/S0140-6736(04)17226-7. [DOI] [PubMed] [Google Scholar]
- Friesema E.C., Kuiper G.G., Jansen J., Visser T.J., Kester M.H. Thyroid hormone transport by the human monocarboxylate transporter 8 and its rate-limiting role in intracellular metabolism. Mol. Endocrinol. 2006;20:2761–2772. doi: 10.1210/me.2005-0256. [DOI] [PubMed] [Google Scholar]
- Fruhbeis C., Frohlich D., Kuo W.P., Amphornrat J., Thilemann S., Saab A.S., Kirchhoff F., Mobius W., Goebbels S., Nave K.A., Schneider A., Simons M., Klugmann M., Trotter J., Kramer-Albers E.M. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11:e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gika A.D., Siddiqui A., Hulse A.J., Edward S., Fallon P., Mcentagart M.E., Jan W., Josifova D., Lerman-Sagie T., Drummond J., Thompson E., Refetoff S., Bonnemann C.G., Jungbluth H. White matter abnormalities and dystonic motor disorder associated with mutations in the SLC16A2 gene. Dev. Med. Child Neurol. 2010;52:475–482. doi: 10.1111/j.1469-8749.2009.03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulburn A.L., Alden D., Davis R.P., Micallef S.J., Ng E.S., Yu Q.C., Lim S.M., Soh C.L., Elliott D.A., Hatzistavrou T., Bourke J., Watmuff B., Lang R.J., Haynes J.M., Pouton C.W., Giudice A., Trounson A.O., Anderson S.A., Stanley E.G., Elefanty A.G. A targeted NKX2.1 human embryonic stem cell reporter line enables identification of human basal forebrain derivatives. Stem Cells. 2011;29:462–473. doi: 10.1002/stem.587. [DOI] [PubMed] [Google Scholar]
- Groeneweg S., Peeters R.P., Visser T.J., Visser W.E. Therapeutic applications of thyroid hormone analogues in resistance to thyroid hormone (RTH) syndromes. Mol Cell Endocrinol. 2017 doi: 10.1016/j.mce.2017.02.029. (EPub Ahead of Print) [DOI] [PubMed] [Google Scholar]
- Hagenbuch B., Meier P.J. The superfamily of organic anion transporting polypeptides. Biochim. Biophys. Acta. 2003;1609:1–18. doi: 10.1016/s0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- Kapoor R., Fanibunda S.E., Desouza L.A., Guha S.K., Vaidya V.A. Perspectives on thyroid hormone action in adult neurogenesis. J. Neurochem. 2015;133:599–616. doi: 10.1111/jnc.13093. [DOI] [PubMed] [Google Scholar]
- Kerr C.L., Letzen B.S., Hill C.M., Agrawal G., Thakor N.V., Sterneckert J.L., Gearhart J.D., All A.H. Efficient differentiation of human embryonic stem cells into oligodendrocyte progenitors for application in a rat contusion model of spinal cord injury. Int J Neurosci. 2010;120:305–313. doi: 10.3109/00207450903585290. [DOI] [PubMed] [Google Scholar]
- Kersseboom S., Horn S., Visser W.E., Chen J., Friesema E.C., Vaurs-Barriere C., Peeters R.P., Heuer H., Visser T.J. In vitro and mouse studies supporting therapeutic utility of triiodothyroacetic acid in MCT8 deficiency. Mol Endocrinol. 2014;28:1961–1970. doi: 10.1210/me.2014-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinne A., Kleinau G., Hoefig C.S., Gruters A., Kohrle J., Krause G., Schweizer U. Essential molecular determinants for thyroid hormone transport and first structural implications for monocarboxylate transporter 8. J. Biol. Chem. 2010;285:28054–28063. doi: 10.1074/jbc.M110.129577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeng P., Decimo D., Pettmann B., Janet T., Labourdette G. Retinoic acid regulates the development of oligodendrocyte precursor cells in vitro. J. Neurosci. Res. 1994;39:613–633. doi: 10.1002/jnr.490390602. [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Petratos S. Thyroid hormone signaling in oligodendrocytes: from extracellular transport to intracellular signal. Mol. Neurobiol. 2016;53:6568–6583. doi: 10.1007/s12035-016-0013-1. [DOI] [PubMed] [Google Scholar]
- Lin L., Yee S.W., Kim R.B., Giacomini K.M. SLC transporters as therapeutic targets: emerging opportunities. Nat. Rev. Drug Discov. 2015;14:543–560. doi: 10.1038/nrd4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Espindola D., Morales-Bastos C., Grijota-Martinez C., Liao X.H., Lev D., Sugo E., Verge C.F., Refetoff S., Bernal J., Guadano-Ferraz A. Mutations of the thyroid hormone transporter MCT8 cause prenatal brain damage and persistent hypomyelination. J. Clin. Endocrinol. Metab. 2014;99:E2799–804. doi: 10.1210/jc.2014-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerl S., Muller J., Bauer R., Richert S., Kassmann C.M., Darras V.M., Buder K., Boelen A., Visser T.J., Heuer H. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J. Clin. Invest. 2014;124:1987–1999. doi: 10.1172/JCI70324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S., Lee X., Hu Y., Ji B., Shao Z., Yang W., Huang G., Walus L., Rhodes K., Gong B.J., Miller R.H., Pepinsky R.B. Death receptor 6 negatively regulates oligodendrocyte survival, maturation and myelination. Nat. Med. 2011;17:816–821. doi: 10.1038/nm.2373. [DOI] [PubMed] [Google Scholar]
- Moog N.K., Entringer S., Heim C., Wadhwa P.D., Kathmann N., Buss C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. 2017;342:68–100. doi: 10.1016/j.neuroscience.2015.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm F.J., Lager A.M., Zaremba A., Wyatt K., Caprariello A.V., Factor D.C., Karl R.T., Maeda T., Miller R.H., Tesar P.J. Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat. Biotechnol. 2013;31:426–433. doi: 10.1038/nbt.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrakhovitch E.A., Olsson P.E., Jiang S., Cherian M.G. Interaction of metallothionein with tumor suppressor p53 protein. FEBS Lett. 2006;580:1235–1238. doi: 10.1016/j.febslet.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Petratos S., Azari M.F., Lee J.Y., Kim M.J. 2016. Improvements in Oligodendroglial Cell Culturing Methods and in Methods for Treating Neurodegenerative Disorders by Using Thyroid Hormones or Analogues. [Google Scholar]
- Pizzagalli F., Hagenbuch B., Stieger B., Klenk U., Folkers G., Meier P.J. Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Mol. Endocrinol. 2002;16:2283–2296. doi: 10.1210/me.2001-0309. [DOI] [PubMed] [Google Scholar]
- Pringle N.P., Yu W.P., Guthrie S., Roelink H., Lumsden A., Peterson A.C., Richardson W.D. Determination of neuroepithelial cell fate: induction of the oligodendrocyte lineage by ventral midline cells and sonic hedgehog. Dev. Biol. 1996;177:30–42. doi: 10.1006/dbio.1996.0142. [DOI] [PubMed] [Google Scholar]
- Roberts L.M., Woodford K., Zhou M., Black D.S., Haggerty J.E., Tate E.H., Grindstaff K.K., Mengesha W., Raman C., Zerangue N. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology. 2008;149:6251–6261. doi: 10.1210/en.2008-0378. [DOI] [PubMed] [Google Scholar]
- Schwartz C.E., May M.M., Carpenter N.J., Rogers R.C., Martin J., Bialer M.G., Ward J., Sanabria J., Marsa S., Lewis J.A., Echeverri R., Lubs H.A., Voeller K., Simensen R.J., Stevenson R.E. Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am. J. Hum. Genet. 2005;77:41–53. doi: 10.1086/431313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirakov M., Skah S., Lone I.N., Nadjar J., Angelov D., Plateroti M. Multi-level interactions between the nuclear receptor TRalpha1 and the WNT effectors beta-catenin/Tcf4 in the intestinal epithelium. PLoS One. 2012;7:e34162. doi: 10.1371/journal.pone.0034162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Suzuki S., Suzuki N., Mori J., Oshima A., Usami S., Hashizume K. micro-Crystallin as an intracellular 3,5,3′-triiodothyronine holder in vivo. Mol. Endocrinol. 2007;21:885–894. doi: 10.1210/me.2006-0403. [DOI] [PubMed] [Google Scholar]
- Takeshige K., Sekido T., Kitahara J., Ohkubo Y., Hiwatashi D., Ishii H., Nishio S., Takeda T., Komatsu M., Suzuki S. Cytosolic T3-binding protein modulates dynamic alteration of T3-mediated gene expression in cells. Endocr. J. 2014;61:561–570. doi: 10.1507/endocrj.ej13-0418. [DOI] [PubMed] [Google Scholar]
- Tamai I., Nezu J., Uchino H., Sai Y., Oku A., Shimane M., Tsuji A. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem. Biophys. Res. Commun. 2000;273:251–260. doi: 10.1006/bbrc.2000.2922. [DOI] [PubMed] [Google Scholar]
- Trajkovic M., Visser T.J., Mittag J., Horn S., Lukas J., Darras V.M., Raivich G., Bauer K., Heuer H. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J. Clin. Invest. 2007;117:627–635. doi: 10.1172/JCI28253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaurs-Barriere C., Deville M., Sarret C., Giraud G., des Portes V., Prats-Vinas J.M., de Michele G., Dan B., Brady A.F., Boespflug-Tanguy O., Touraine R. Pelizaeus-Merzbacher-Like disease presentation of MCT8 mutated male subjects. Ann. Neurol. 2009;65:114–118. doi: 10.1002/ana.21579. [DOI] [PubMed] [Google Scholar]
- Verge C.F., Konrad D., Cohen M., Di Cosmo C., Dumitrescu A.M., Marcinkowski T., Hameed S., Hamilton J., Weiss R.E., Refetoff S. Diiodothyropropionic acid (DITPA) in the treatment of MCT8 deficiency. J. Clin. Endocrinol. Metab. 2012;97:4515–4523. doi: 10.1210/jc.2012-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser T.J. Thyroid hormone transporters and resistance. Endocr. Dev. 2013;24:1–10. doi: 10.1159/000343695. [DOI] [PubMed] [Google Scholar]
- Visser W.E. Triac Trial in MCT8 Patients. 2014. Erasmus Medical Center.https://clinicaltrials.gov/ct2/show/NCT02060474 [Online]. Clinicaltrials.gov. Available. [Accessed] [Google Scholar]
- Visser W.E., Friesema E.C., Jansen J., Visser T.J. Thyroid hormone transport in and out of cells. Trends Endocrinol. Metab. 2008;19:50–56. doi: 10.1016/j.tem.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Watkins T.A., Emery B., Mulinyawe S., Barres B.A. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron. 2008;60:555–569. doi: 10.1016/j.neuron.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth E.K., Roth S., Blechschmidt C., Holter S.M., Becker L., Racz I., Zimmer A., Klopstock T., Gailus-Durner V., Fuchs H., Wurst W., Naumann T., Brauer A., de Angelis M.H., Kohrle J., Gruters A., Schweizer U. Neuronal 3′,3,5-triiodothyronine (T3) uptake and behavioral phenotype of mice deficient in Mct8, the neuronal T3 transporter mutated in Allan-Herndon-Dudley syndrome. J. Neurosci. 2009;29:9439–9449. doi: 10.1523/JNEUROSCI.6055-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosik K., Antel J., Kuhlmann T., Bruck W., Massie B., Nalbantoglu J. Oligodendrocyte injury in multiple sclerosis: a role for p53. J. Neurochem. 2003;85:635–644. doi: 10.1046/j.1471-4159.2003.01674.x. [DOI] [PubMed] [Google Scholar]
- Zada D., Tovin A., Lerer-Goldshtein T., Vatine G.D., Appelbaum L. Altered behavioral performance and live imaging of circuit-specific neural deficiencies in a zebrafish model for psychomotor retardation. PLoS Genet. 2014;10:e1004615. doi: 10.1371/journal.pgen.1004615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaka M., Rafi M.A., Rao H.Z., Luzi P., Wenger D.A. Insulin-like growth factor-1 provides protection against psychosine-induced apoptosis in cultured mouse oligodendrocyte progenitor cells using primarily the PI3K/Akt pathway. Mol. Cell. Neurosci. 2005;30:398–407. doi: 10.1016/j.mcn.2005.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material