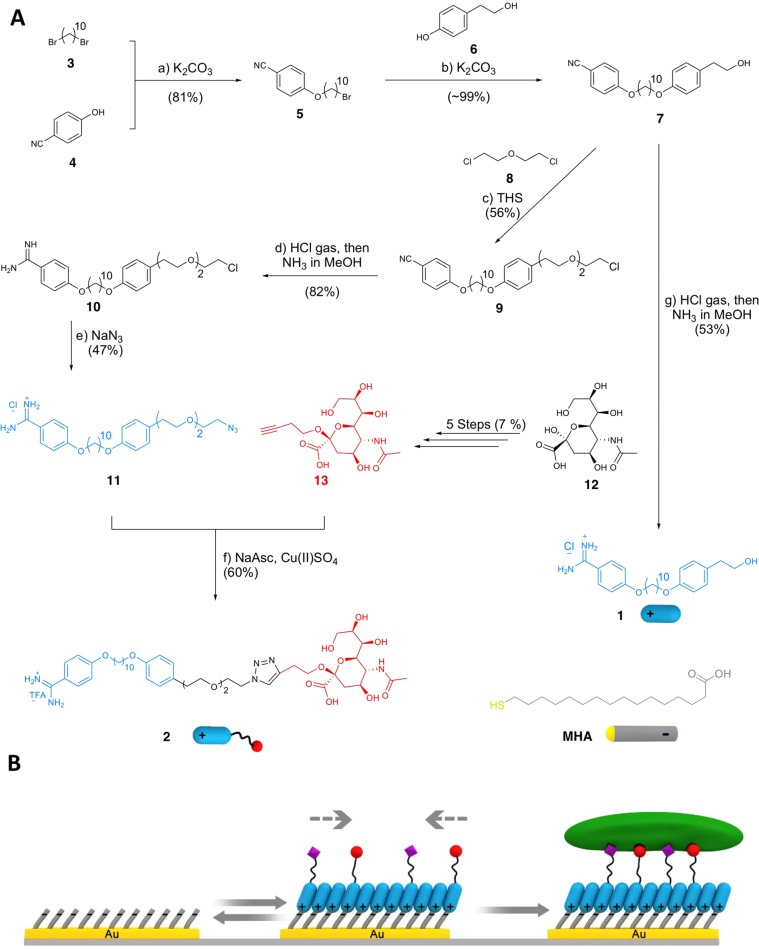
Figure 1.
(A) Synthetic pathway of OH-terminated amphiphile 1 and sialic acid terminated amphiphile 2 and (B) use of 1 and 2 to form an adaptable rSAM. Reagents and conditions in panel A: (a) 1,10-dibromodecane 3 10 equiv, K2CO3 2 equiv, acetone, 80 °C, 24 h, 81%; (b) 4-(2-hydroxyethyl)phenol 6 2.0 equiv, K2CO3 2.0 equiv, acetone, 80 °C, 24 h, ∼99%; (c) 2-chloroethyl ether 8 43 equiv, tetrabutylammonium hydrogen sulfate (THS) 2.0 equiv, NaOH solution (50% w/w), rt, 18 h, 56%; (d) HCl gas, MeOH, 0 °C → rt, 24 h, then NH3 in MeOH, rt, 24 h, 82%; (e) NaN3 4.0 equiv, DMF, 90 °C, 24 h, 47%; (f) NaAsc 3.0 equiv, Cu(II)SO4 0.3 equiv, H2O/2-butanol (1:2), rt, 4 h, 60%; (g) HCl gas, 1,4-dioxane, MeOH, 0 °C → rt, 24 h, then NH3 in MeOH, rt, 24 h, 53%.