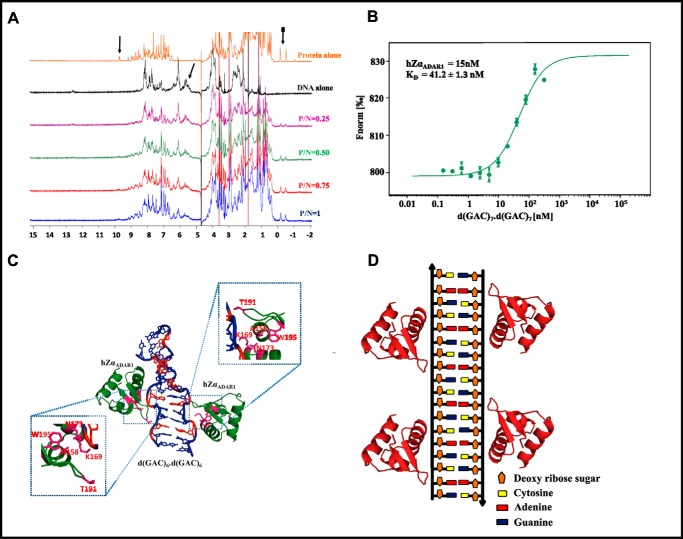
Figure 7.
D(GAC)7·d(GAC)7–hZαADAR1 complex model. A, 1H NMR spectra corresponding to d(GAC)7·d(GAC)7 duplex titration with hZαADAR1. Arrows indicate the reduction in peak intensities (peak broadening) as the concentration of protein increases, suggestive of an intermediate chemical exchange between the two. B, DNA concentration–dependent (the protein concentration is kept constant, whereas the DNA concentration is varied, as described under “Experimental Procedures”) binding isotherms obtained from the microscale thermophoresis assay indicate that d(GAC)7·d(GAC)7 and hZαADAR1 exhibit nanomolar binding affinity with a KD of 41 nm. C, 1H NMR–based docked model of hZαADAR1 (PDB code 2ACJ)–d(GAC)6·d(GAC)6 (MD-derived) complex (red represents the A … A mismatch). The important interactions are enlarged and boxed. D, schematic of hZαADAR1 binding at multiple mismatch sites of the d(GAC)6·d(GAC)6 duplex.