Figure 2.
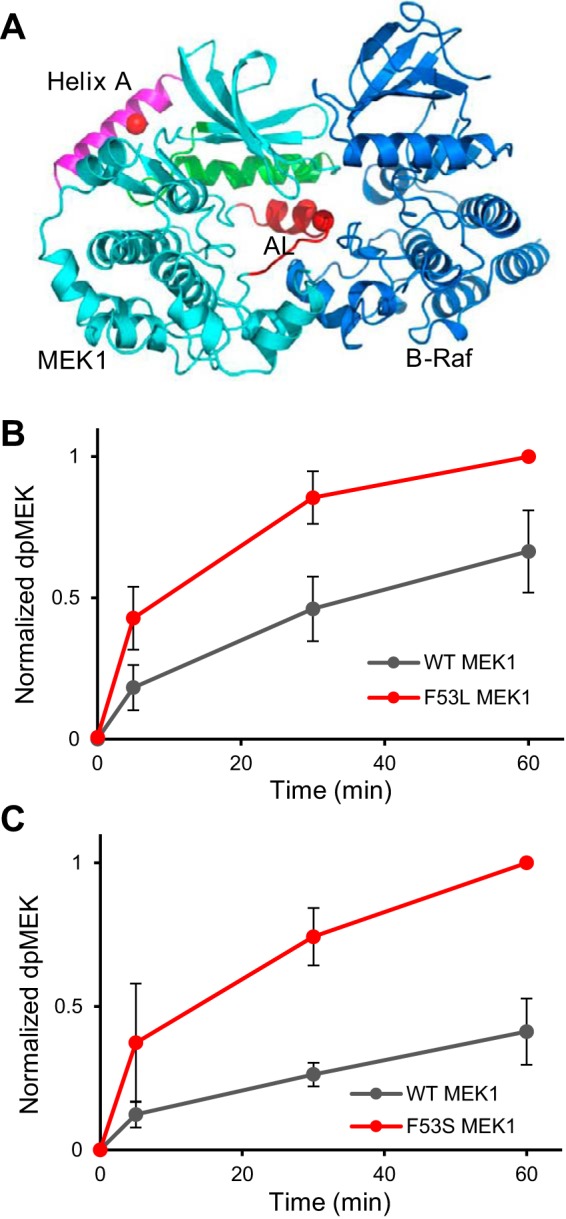
Activating mutations enhance the rate of MEK1 phosphorylation by Raf. A, complex between B-Raf and MEK1 is based on a structure (PDB code 4mne), with helix A modeled from PDB code 5bx0 (a more complete structure of MEK1). B and C, phosphorylation of MEK1/F53L and MEK1/F53S occurs faster than phosphorylation of wild-type MEK1. The normalized dpMEK is an average of three experimental replicates. The reaction was run at a Raf/MEK ratio of 1:∼217. Error bars, S.E.
