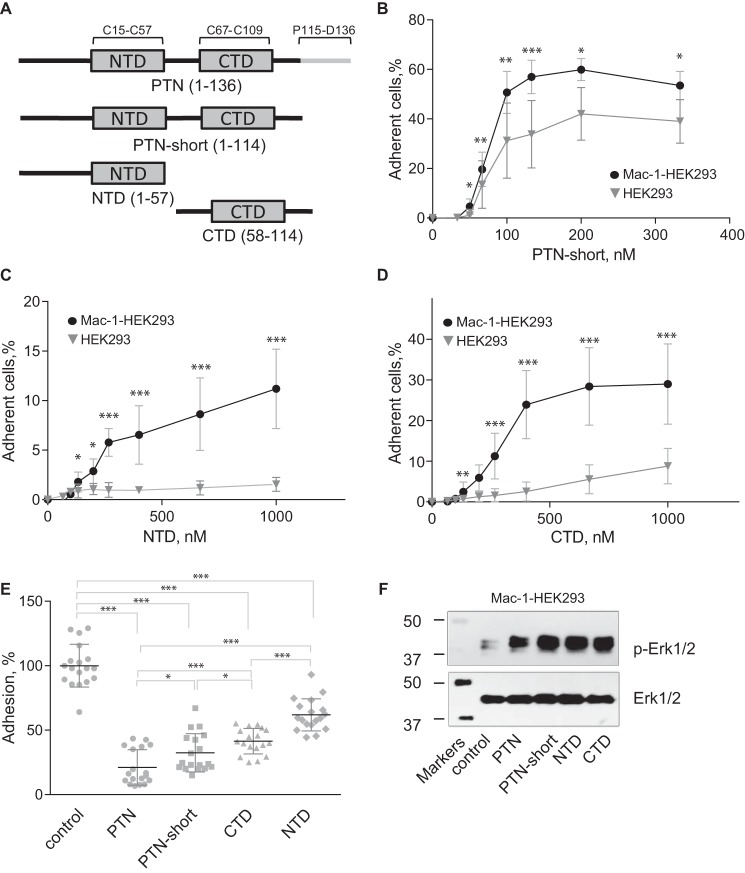
Figure 8.
PTN fragments support adhesion, inhibit adhesion, and induce Erk1/2 activation in Mac-1–expressing HEK293 cells. A, a schematic representation of the domain structure of PTN and recombinant fragments used in the adhesion and Erk1/2 activation assays. B, aliquots (100 μl; 5 × 104/ml) of Mac-1 HEK293 and wild-type HEK293 cells labeled with calcein were added to microtiter wells coated with different concentrations of truncated PTN without the C-terminal tail (PTN-short) and postcoated with 1% PVP. After 30 min at 37 °C, nonadherent cells were removed by washing, and fluorescence of adherent cells was measured. Data shown are means ± S.E. from three separate experiments with triplicate measurements. Error bars represent S. E. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001. C and D, adhesion assays with immobilized NTD (C) and CTD (D) were performed as described in B. E, Mac-1 HEK293 cells were preincubated with 5 μm PTN or PTN fragments for 20 min before loading into wells coated with 100 nm PTN and postcoated with 1% PVP. Adhesion in the absence of soluble PTN or PTN fragments was assigned a value of 100%. Data shown are means ± S.E. from three separate experiments with sextuplicate measurements. Error bars represent S. E. *, p ≤ 0.05; ***, p ≤ 0.001 compared with control adhesion in the absence of inhibitors. F, Mac-1 HEK293 cells were treated with 0.7 μm PTN or PTN fragments. After 30 min at 37 °C, cells were lysed, and lysates were separated by 12.5% SDS-PAGE. Western blotting was performed using the mAb specific for the phosphorylated form of Erk1/2 (p-Erk1/2). The mAb against total Erk1/2 was used to equalize protein loading. A representative of three experiments is shown.