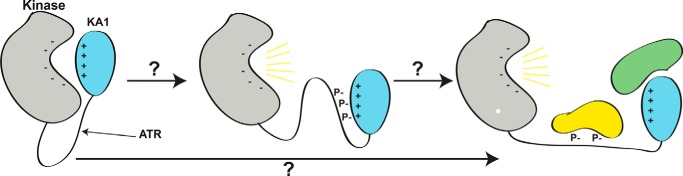
Figure 6.
A proposed model for the role of linker phosphorylation in Chk1 activation. It is not known how phosphorylation of the linker region between kinase (gray) and KA1 (cyan) domains of Chk1 by ATR results in release of autoinhibition and full activation of Chk1 (yellow lines), potentially assisted by additional binding partners (lower arrow, green and yellow proteins). Due to the reliance of KA1-mediated autoinhibition of Chk1 on basic residues of CM1 and CM2, we propose that phosphorylation of the linker region by ATR and subsequent autophosphorylation may interrupt the charge–charge interaction between KA1 and kinase domain by binding to CM1 and CM2 with negatively charged phosphates (middle panel). This conformation could activate Chk1 on its own, or act as an intermediate between the autoinhibited state and sustained activation through binding other partners at sites of Chk1 phosphorylation or the KA1 domain itself.