Abstract
The aim of this study was to standardize and investigate the changes in corneal clarity with age. Densitometry software for the Oculus Pentacam was used to examine corneal clarity at different age groups.
A total of 192 eyes from 97 healthy participants were included in this cohort comparative nonrandomized, cross-sectional study. An Oculus Pentcam was used to image the cornea of healthy participants grouped by age (between 10 and 70 years old). Data from the densitometry output have been used to determine clarity in concentric zones and different depths of the cornea.
Corneal densitometry (CD) across all ages showed significant differences between groups when divided into the following layers: anterior, central, and posterior or divided into 0 to 2, 2 to 6, and 6 to 10 mm concentric zones (P < .05). The most striking decrease in clarity occurred with age in all 3 layers of the periphery (6–10 mm) (P < .05). In addition, we showed that the 10 to 19-year age group had lower clarity than the 20 to 30-age group (P < .05), and after 30 years, the cornea shows a steady progression of increased or decreased clarity.
The values for CD, as well as for separate subdivisions based on layer and surface area, might provide a standard for use in further studies and clinical practice. This study established that relation between CD and age is differed when the cornea is divided into layers and zones. This study suggests that there are other factors that may play an essential role in corneal clarity as well as age.
Keywords: apoptosis, cornea, densitometry, keratocyte, stroma
1. Introduction
Corneal stromal transparency relies on the organized collagen fibrils arranged in parallel to form lamellae resulting in no or very minimal light scattering.[1] For both sclera and cornea, collagen is termed as the fundamental structural constituent. Its intense tensile strength is capable of facilitating the globe with a flexible, shielding coat. The stroma and Bowman layer essentially house the corneal collagen, with the former region comprising of 90% of a hydrated cornea's entire thickness. Going by the arguments stipulated,[2] the corneal stroma is composed of a vastly structured arrangement of small-diameter collagen fibrils supported apart by a proteoglycan matrix, which ensure uniformity in inter-fibrillar spacing.
The posterior corneal endothelium and anterior external corneal epithelial cell layer are the key light scattering sources.[1] The regular cornea disperses light primarily at the tear film-cornea interface and at the air-tear film, just as anticipated from an optically transparent tissue, where the light refraction's index change is presumably maximum with slight interior structures (cornea tissue) scattering, such as cell nuclei and nerves. Due to its uniform arrangement of collagen fibrils, the corneal stroma is able to sustain its clarity in a network-type formation in lamellar sheets[1,3–5]; this clarity is highly influenced by both the collagen fibrils’ size and fibrils’ spacing within the configuration.[5]
In the endothelium, specific augmentation in cellular pleomorphism and polymegethism happens, with a consequent decrease of cell quantity with age. In addition to such age-affiliated variations is the loss of the cornea's initial regular hexagonal outline and no noteworthy vertical regional discrepancy or inconsistency among paired corneas in cellular pleomorphism or polymegethism exist in the typical corneas analyzed.[6]
A corresponding increase in the fibrils’ cross-sectional area is witnessed with increasing age owing to the progressive collagen deposition.[7] Both functional and structural variations are produced in corneal aging. Such alterations can eventually influence the aptitude of the eye to repair itself, refract light, and protect the internal structures.[8]
The human cornea is characterized by changeable thickness (CCT), aspheric curvature, as well as being anisotropic, meaning it is capable of exhibiting diverse physical qualities upon application of stress in various directions. Such properties are variable, varying with progressive age, corneal pathology, and the degree of hydration whereby a loss of lamellae structuring leads to varied corneal biomechanics.[9] Clarity failure is the innate reaction of cornea to an extensive variety of pathological problems such corneal dystrophies, infections, and degeneration. Analysis and assessment of the resultant corneal haze is hence a fundamental constituent of ophthalmological assessment. Medically, this is normally done by a typical slit-lamp assessment with documentation of results, which can be supplemented with an explanatory severity scale.[10]
The Oculus Pentacam (OCULUS Optikgerate GmbH, Wetzlar, Germany) designed on the Schiempflug photography principle is a noninvasive camera, designed to capture images of the anterior segment of the eye and to produce a comprehensive analysis of the cornea and its densitometry. A better knowledge of corneal clarity in normal control subjects with no known eye disease will help us to understand the normal changes in corneal clarity with age. To date, there is no such study that includes measurements from juvenile corneas. Such data may help when planning treatments or measuring how beneficial treatments have been in patients with corneal disease. This study compared the corneal densitometry (CD) measurements in healthy volunteer participants within different age groups.
2. Methods
2.1. Participants and densitometry measurements
A prospective comparative and nonrandomized cross-sectional study was approved by Central Manchester University Hospitals NHS Foundation Trust, Manchester, UK, and Health Research Authority by NREC local ethics committee. Written consent was taken from the subjects before collecting their data. All participants were healthy and had no ocular pathology other than refractive error and were all above 10 years of age at the time of imaging.
The corneas of normal controls, with no known corneal disease, were photographed with the Oculus Pentacam. In order to collect the images, participants were positioned in front of the Pentacam camera with their chin and forehead resting on the frame. CD was determined using the pentacam densitometry software. The data extracted from images of the controls’ eyes were categorized into 6 age groups (10–19, 20–29, 30–39, 40–49, 50–59, and 60–69 age groups). For analysis, corneas were split into 3 concentric zones 0 to 2, 2 to 6, and 6 to 10 mm and also subdivided into anterior 120 μm, central, and posterior 60 μm layers.
2.2. Statistics
A data analysis was carried out using IBM SPSS statistics software package v.23 for MAC; Armonk, NY: IBM Corp. Descriptive statistics were presented as the mean ± SD. Normality of data was examined using Kolmogorov–Smirnov test. Student t test for 2 independent samples was used when parametric analysis was possible and the nonparametric of Mann–Whitney U test was applied when parametric analysis was not possible. Person correlation and regression line were used to look at correlation statistics between age and CD. One-way analysis of variance (ANOVA) was used for comparing more than 2 groups. A P value of < .05 was considered to be statistically significant.
IBM SPSS Statistics for Mac, Version 23.0. Armonk, NY: IBM Corp
3. Results
A total of 192 eyes (97 right eyes, 95 left eyes) from 97 healthy participants (36.08% male, 63.91% female) were included in this study that were distributed over 6 age groups. Participant's age varied between 10 and 69 years. The mean age of the controls was 36.15 years with a SD of 16.5. Female average age was 36.07 years with SD 16.78, while the average age of the male group was 36.12 years with SD 17.45. The demographics of the controls are summarized in Table 1 and shows that there was no diversity between male and female participants.
Table 1.
Demographics of the subjects.

The mean CD results are summarized in Table 2. CD across all age groups differed significantly between groups when divided both into anterior, central, or posterior layers or analyzed in full depth. There were also significant differences when the cornea was divided into concentric zones of 0 to 2, 2 to 6, and 6 to 10 mm or when analyzed as the total corneal diameter 0 to 10.
Table 2.
Corneal densitometry mean (SD) and P value across the 6 age groups for different corneal zones and layers.

Corneal clarity in the anterior layer across all zones was found to be low in the 10 to 19 age groups. The clarity improved in the 20 to 29 age groups before it started to decrease in the 40 to 49 age groups, the most significant being the 0 to 2 mm and 2 to 6 mm zones, which are clearer at 20 to 29 years before the densitometry values starting to be increased with age. The anterior layer at zone 0 to 2 mm has better clarity in the 60 to 69 age groups. CD in the central layer increases with age across all corneal zones. CD in the posterior layer also increases with age across all concentric zones. Once again, the posterior 6 to 10 mm shows a rapid increase with age. Figure 1 shows the changes over each layer across different corneal layer zones.
Figure 1.
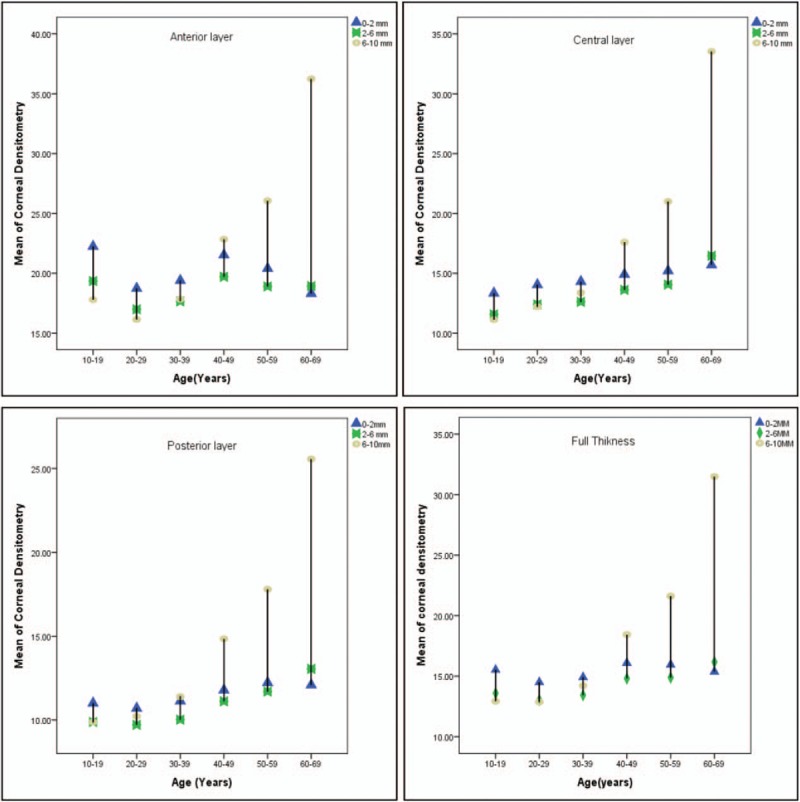
Diversity in corneal densitometry take place in different corneal layers at different age groups.
Age was found to be significantly correlated with CD at all layers zone when we look at all the total diameter of 0 to 10 mm. In addition, a significant correlation was found for all corneal zone and layers except the anterior 0 to 2 mm, zone which were not statistically significant. Table 3 and Fig. 2 give details of this relationship.
Table 3.
Person correlation between age and corneal densitometry in different corneal layer and zone.

Figure 2.
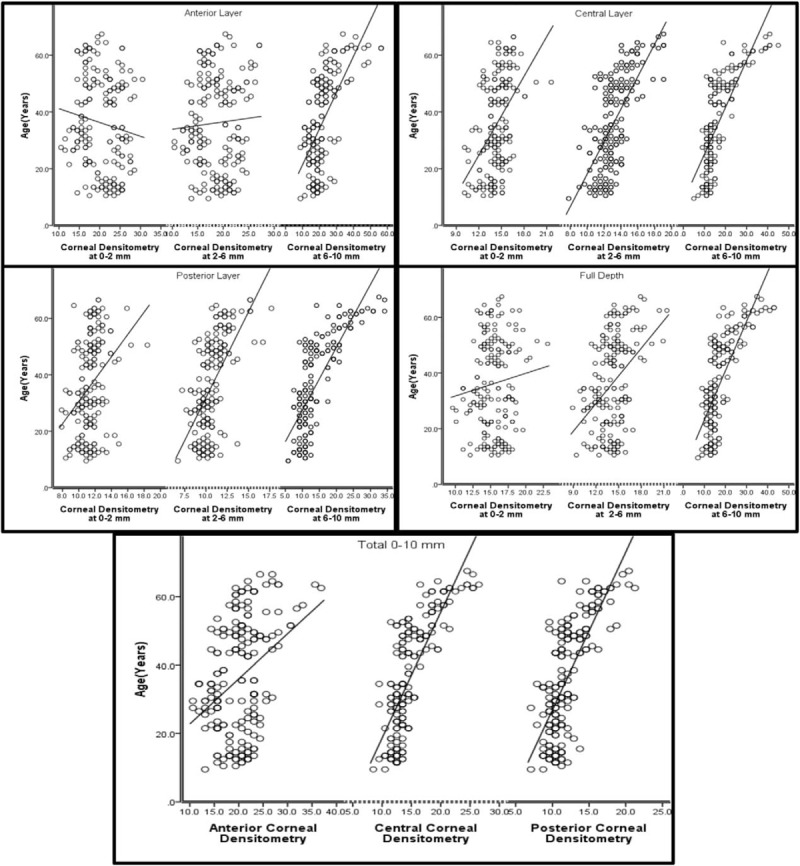
Relation between age and corneal densitometry in different layers and zones.
4. Discussion
This study demonstrates that corneal clarity decreases with age particularly in the 6 to 10 mm concentric zone. One possible reason for this decrease in clarity may be changes that occur in the corneal endothelium. Endothelial changes related to age have been studied both experimentally and clinically. It has been demonstrated that between the ages of 20 and 80, the yearly reduction in cell density of the corneal endothelium is about 0.6%, with concomitant increases in pleomorphism and polymegethism.[8,11–14] These age-related changes to the endothelium lead to increases in corneal thickness and may increase CD measurements.
Due to the fact that the cornea is the scaffold for the major refractive surface of the eye, any biological or mechanical response to injury will also affect optical performance. The same mechanisms responsible for preserving ocular integrity can, as a result, undermine the goals of accomplishing stable and predictable visual results after refractive procedures.[15] One report[16] discovered a significant early rise in the mean anterior density readings (from 27.71 ± 4.39 to 37.812 ± 12.31) 3 months after photorefractive keratectomy (PRK) that subsequently reduced again by 12 months (26.291 ± 4.93). The high CD at 3 months is related to the wound healing process. Activated keratocyte apoptosis was found to be higher post-PRK; these cells were located in the anterior stroma causing haze that improved over time.[17]
This study found a fluctuation of densitometry values in both 0 to 2 and 2 to 6 mm zone in the anterior layer at different age groups. Ten to 19 years and 40 to 49 years age groups were found to have higher CD. These 2 age groups are where significant hormonal changes can take place in the human body. CCT changes in women have been reported previously during pregnancy[18] and during the menstrual cycle. [19] It has been reported that contact lens (CL) use during the premenstrual phase is easier than during the menstrual period.[20,21] This may be due to changes in CCT leading to changes of corneal curvature,[22] which can cause intolerance to CL wear. In addition, the lack of sex hormones has been suggested as a possible reason for changes in the corneal biomechanics of individuals over the age of 40.[23] It is therefore possible that hormones may also play a role in the changes in corneal clarity that this study found with age.
Corneal remodeling reported to take place in keratoconus (KCN),[24] postrefractive ectasia,[25] and in injured stroma[26] all lead to increases in corneal haze. This remodeling has been suggested to play a role in the relationship between anterior cornea changes and visual acuity (VA) after corneal crosslinking (CXL)[27,28] or corneal trauma.[29] Corneal remolding activity may be able to explain why the teenage group in this study has higher levels of CD than those of the next age group (20–29), as the keratocyte activity is known to be higher in this younger group.
The cornea is the major physical covering of the eye. To contribute to the focusing ability of the eye, it has to be tough, transparent, and capable of maintaining a smooth and steady curvature. Controversies still exist over how transparency is maintained even with the advanced understanding of the structure and other properties of the cornea. One area of interest is to investigate the role of hydration, which is a major determinant of transparency and which can be manipulated within reasonable parameters under standardized conditions.[3,30,31] The studies[32] of corneal backscatter established that there was a remarkable growth in total CD with advancing age. In contrast, it has also been shown that CD increases in the central 6 mm of the cornea are minimal with respect to age. In the current study, it was shown that, whereas there is no age-linked variation in the central 6 mm of the cornea, a significant rise is seen in the region next to the limbus. The study excluded the 10 to 12 mm area zone from analysis in our study because this area could be part of the limbus with various changes in the white-to-white thickness of the cornea,[33] suggesting that some individuals have corneas with analysis parameters smaller than 12 mm. The higher backscatter quantities therefore occur when sclera and parts of the limbus are involved in the assessment of the peripheral layers. Even slight variations in the situation of the limbus would physically influence the 10 to 12 mm CD outcomes. This, however, does not take place in the significantly dominant annuli. Owing to this factor, the substantial expansion in scatter in the 10 to 12 mm zone can neither be absolutely understood nor rightly correlated with age.[34]
All the measured limits were connected with each other, signifying that the scattering for every region of the cornea is constant with respect to other portions of the cornea. It is interesting, therefore, that a statistically substantial connection between the difference in VA, stromal scattering, and assessed interface was established. This indicates that the complete scattering capacities may not be as significant in influencing VA as is their change throughout the cornea.[35]
When this study looks at correlation between total CD and age, we found it is correlated positively. Earlier studies[36,37] revealed that light scatter in the cornea is a phenomenon that is related to age and that the best preoperative forecaster of postoperative VA is age. Consequently, the ages of patients in the study might have an effect on the dimensions of the scattering that are determined from Pentacam images.
In summary, our study findings agree with other study,[38] as there was a considerable increase in CD with age, despite the fact that the increase was confined to the peripheral cornea. Conversely, this study finds a relationship between certain age groups and CD, notably within the teenage age group and those within the 40 to 49 age group. A possible explanation for this observation may be the hormonal changes known to occur at this time. Interestingly, that teen-age group was found to be cloudier than 20 to 29 age groups, suggesting that the additional factor of corneal remodeling with increased keratocyte activity may be relevant.
This study was limited by small sample size. However, this study is first study to our knowledge that aims to standardized CD value in healthy control eyes to include those individuals aged between 10 and 19 years. This study suggests that additional factors other than age alone may have a role to play in corneal clarity. These values for CD dimensions, as well as subdivisions based on layer and surface area, might provide a standardized stage for use in further studies and clinical practice.
Acknowledgment
The authors thank the Manchester royal Eye hospital (corneal clinic) for supporting patient's recruitments for this study.
Footnotes
Abbreviations: CCT = corneal thickness, CD = corneal densitometry, CL = contact lens, CXL = corneal crosslinking, KCN = keratoconus, PRK = photorefractive keratectomy, VA = visual acuity.
Funding/support: This study was supported by Armed Forces Medical Services, Ministry of Defence, Riyadh, Saudi Arabia.
The authors report no conflicts of interest.
References
- [1].Maurice DM. The structure and transparency of the cornea. J Physiol 1957;136:263–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Maurice DM (1988) Mechanics of the cornea, in The Cornea: Transactions of the World Congress on the Cornea III, Cavanagh HD, Editor. New York: Raven Press. p. 187-193. [Google Scholar]
- [3].Freegard TJ. The physical basis of transparency of the normal cornea. Eye 1997;11:465–71. [DOI] [PubMed] [Google Scholar]
- [4].Qazi Y, Wong G, Monson B, et al. Corneal transparency: genesis, maintenance and dysfunction. Brain Res Bull 2010;81:198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Borcherding MS, Blacik L, Sittig R, et al. Proteoglycans and collagen fibre organization in human corneoscleral tissue. Exp Eye Res 1975;21:59–70. [DOI] [PubMed] [Google Scholar]
- [6].Yee RW, Matsuda M, Schultz RO, Edelhauser HF. Changes in the normal corneal endothelial cellular pattern as a function of age. Curr Eye Res 1985;4:671–8. [DOI] [PubMed] [Google Scholar]
- [7].Daxer A, Misof K, Grabner B, et al. Collagen fibrils in the human corneal stroma: structure and aging. Invest Ophthalmol Vis Sci 1998;39:644–7. [PubMed] [Google Scholar]
- [8].Faragher R, Mulholland B, Tuft S, et al. Aging and the cornea. Br J Ophthalmol 1997;81:814–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kotecha A. What biomechanical properties of the cornea are relevant for the clinician? Survey Ophthalmol 2007;52:S109–14. [DOI] [PubMed] [Google Scholar]
- [10].Braunstein RE, Jain S, McCally RL, et al. Objective measurement of corneal light scattering after excimer laser keratectomy. Ophthalmology 1996;103:439–43. [DOI] [PubMed] [Google Scholar]
- [11].Blatt HL, Rao GN, Aquavella JV. Endothelial cell density in relation to morphology. Invest Ophthalmol Vis Sci 1979;18:856–9. [PubMed] [Google Scholar]
- [12].Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial cell changes over a ten-year period. Invest Ophthalmol Vis Sci 1997;38:779–82. [PubMed] [Google Scholar]
- [13].Laule A, Cable MK, Hoffman CE, Hanna C. Endothelial cell population changes of human cornea during life. Arch Ophthalmol 1978;96:2031–5. [DOI] [PubMed] [Google Scholar]
- [14].Murphy C, Alvarado J, Juster R, Maglio M. Prenatal and postnatal cellularity of the human corneal endothelium. A quantitative histologic study. Invest Ophthalmol Vis Sci 1984;25:312–22. [PubMed] [Google Scholar]
- [15].Dupps WJ, Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res 2006;83:709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cennamo G, Forte R, Aufiero B, La Rana A. Computerized Scheimpflug densitometry as a measure of corneal optical density after excimer laser refractive surgery in myopic eyes. J Cataract Refract Surg 2011;37:1502–6. [DOI] [PubMed] [Google Scholar]
- [17].Mohan RR, Hutcheon AE, Choi R, et al. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res 2003;76:71–87. [DOI] [PubMed] [Google Scholar]
- [18].Weinreb RN, Lu A, Beeson C. Maternal corneal thickness during pregnancy. Am J Ophthalmol 1988;105:258–60. [DOI] [PubMed] [Google Scholar]
- [19].Leach N, Wallis N, Lothringer L, Olson J. Corneal hydration changes during the normal menstrual cycle: a preliminary study. J Reprod Med 1971;6:201–4. [PubMed] [Google Scholar]
- [20].Guttridge NM. Changes in ocular and visual variables during the menstrual cycle. Ophthal Physiol Optics 1994;14:38–48. [DOI] [PubMed] [Google Scholar]
- [21].Serrander A, Peek K. Changes in contact lens comfort related to the menstrual cycle and menopause. A review of articles. J Am Optom Assoc 1993;64:162–6. [PubMed] [Google Scholar]
- [22].Kiely PM, Carney LG, Smith G. Menstrual cycle variations of corneal topography and thickness. Am J Optom Physiol Opt 1983;60:822–9. [DOI] [PubMed] [Google Scholar]
- [23].Goto T, Klyce SD, Zheng X, et al. Gender-and age-related differences in corneal topography. Cornea 2001;20:270–6. [DOI] [PubMed] [Google Scholar]
- [24].Silverman RH, Urs R, RoyChoudhury A, et al. Epithelial remodeling as basis for machine-based identification of keratoconus identifying keratoconus based on epithelial remodeling. Invest Ophthalmol Vis Sci 2014;55:1580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rocha KM, Perez-Straziota CE, Stulting RD, Randleman JB. SD-OCT analysis of regional epithelial thickness profiles in keratoconus, postoperative corneal ectasia, and normal eyes. J Refract Surg 2013;29:173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Utsunomiya T, Hanada K, Muramatsu O, et al. Wound healing process after corneal stromal thinning observed with anterior segment optical coherence tomography. Cornea 2014;33:1056–60. [DOI] [PubMed] [Google Scholar]
- [27].Hafezi F. Significant visual increase following infectious keratitis after collagen cross-linking. J Refract Surg 2012;28:587–8. [DOI] [PubMed] [Google Scholar]
- [28].El Saadany AKI, Al-Balkini MS, El Sawy MFAH, Ananian MFB. Scheimpflug camera changes after cross-linking for keratoconus. Menoufia Med J 2015;28:168. [Google Scholar]
- [29].Kanellopoulos AJ, Asimellis G. Corneal epithelial remodeling following cataract surgery: three-dimensional investigation with anterior-segment optical coherence tomography. J Refract Surg 2014;30:348–53. [DOI] [PubMed] [Google Scholar]
- [30].Candia O. Fluid and electrolyte transport in corneal transparency. Mt Sinai J Med 1980;47:74–9. [PubMed] [Google Scholar]
- [31].Duane T. The steady state of corneal hydration. Am J Ophthalmol 1949;32:203–7. [DOI] [PubMed] [Google Scholar]
- [32].Olsen T. Light scattering from the human cornea. Invest Ophthalmol Vis Sci 1982;23:81–6. [PubMed] [Google Scholar]
- [33].Cakmak HB, Cagil N, Simavli H, Raza S. Corneal white-to-white distance and mesopic pupil diameter. Int J Ophthalmol 2012;5:505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hillenaar T, Cals RH, Eilers PH, et al. Normative database for corneal backscatter analysis by in vivo confocal microscopy. Invest Ophthalmol Vis Sci 2011;52:7274–81. [DOI] [PubMed] [Google Scholar]
- [35].Srikumaran D, Munoz B, Aldave AJ, et al. Long-term outcomes of Boston type 1 keratoprosthesis implantation: a retrospective multicenter cohort. Ophthalmology 2014;121:2159–64. [DOI] [PubMed] [Google Scholar]
- [36].Patel SV, Winter EJ, McLaren JW, Bourne WM. Objective measurement of backscattered light from the anterior and posterior cornea in vivo. Invest Ophthalmol Vis Sci 2007;48:166–72. [DOI] [PubMed] [Google Scholar]
- [37].Patel SV, Baratz KH, Hodge DO, et al. The effect of corneal light scatter on vision after Descemet stripping with endothelial keratoplasty. Arch Ophthalmol 2009;127:153–60. [DOI] [PubMed] [Google Scholar]
- [38].Dhubhghaill SN, Rozema JJ, Jongenelen S, et al. Normative values for corneal densitometry analysis by Scheimpflug optical assessment corneal densitometry in the normal population. Invest Ophthalmol Vis Sci 2014;55:162–8. [DOI] [PubMed] [Google Scholar]


