Abstract
Rationale:
Narcolepsy is a rare sleep disorder with disrupted sleep-architecture. Clinical management of narcolepsy lies dominantly on symptom-driven pharmacotherapy. The treatment role of repetitive transcranial magnetic stimulation (rTMS) for narcolepsy remains unexplored.
Patient concerns:
In this paper, we present a case of a 14-year-old young girl with excessive daytime sleepiness (EDS), cataplexy and hypnagogic hallucinations.
Diagnoses:
After excluding other possible medical conditions, this patient was primarily diagnosed with narcolepsy.
Interventions:
The patient received 25 sessions of high-frequency rTMS over the left dorsolateral prefrontal cortex (DLPFC).
Outcomes:
The symptoms of EDS and cataplexy significantly improved after rTMS treatment. Meanwhile, her score in the Epworth sleep scale (ESS) also remarkably decreased.
Lessons:
This case indicates that rTMS may be selected as a safe and effective alternative strategy for treating narcolepsy-like symptoms. Well-designed researches are warranted in future investigations on this topic.
Keywords: adolescent, narcolepsy, repetitive transcranial magnetic stimulation
1. Introduction
Narcolepsy, a chronic neurologic disorder, severely disrupts sleep-wake architecture in affected patients. Typically, its clinical manifestations consist of EDS, cataplexy, sleep paralysis, hypnagogic or hypnopompic hallucinations, and disturbed nocturnal sleep.[1] Narcolepsy with and without cataplexy is respectively known as narcolepsy type 1 and narcolepsy type 2.[2]
Etiological evidence supports that loss of hypocretin-producing neurons in lateral hypothalamus is closely related with narcolepsy type 1.[3] Recent studies have further revealed an immunologic basis of narcolepsy, such as HLA-DQB1×0602 positivity, autoantibodies to hypocretin-producing neurons, and occurrence of this disease following streptococcus infection and H1N1 vaccination.[4–6] Of note, complete loss of F-waves was observed during cataleptic attack, which reflected an alteration of neural firing activity in narcoleptic individuals.[7] In addition, the neural correlates in narcoleptic patients have also been explored with different neuroimaging techniques.[8–10] Using the 3-dimensional surface-based method, localized thinning of cortexes in both hemispheres, including the orbitofrontal gyri, dorsolateral/medial prefrontal cortices, middle/inferior temporal gyri, insula, cingulate gyri, and inferior parietal lobule, was observed in narcoleptic patients with cataplexy.[8] Another study investigated the cerebral perfusion by brain single photon emission computed tomography (SPECT) in narcoleptics, and found hypoferfusion in bilateral cortical areas, including parts of the dorsolateral/ventromedial prefrontal cortices, parahippocampal gyri, cingulate gyri, anterior hypothalami, caudate nuclei, and pulvinar nuclei of thalami.[9] All these findings indicated brain structural and functional abnormalities that contributed to the pathogenesis of narcolepsy.[8–10]
As lack of specific cure, pharmacotherapy for narcolepsy is fundamentally symptom-driven and lifestyle adjustment is emphasized.[11] Therefore, new treatment strategy with favorable adherence, efficacy, and tolerability is urgently needed. rTMS is a noninvasive neuro-modulation method for various neuropsychiatric disorders (such as stroke, epilepsy, depression, anxiety disorder, and obsessive-compulsive disorder), and can be applied to modify the cortical excitability.[12,13] Recently, several studies have used TMS to evaluate the cortical excitability in narcoleptic patients and reported a reduction in cortical excitability, which was further interpreted as a possible cause of EDS.[14–16] However, the therapeutic role of rTMS for narcolepsy has not been investigated yet.
Herein, we presented a patient with narcolepsy-like symptoms, who was successfully treated with high-frequency rTMS over the left dorsolateral prefrontal cortex (DLPFC). With venlafaxine monotherapy to continue her treatment, remission in symptoms of narcolepsy maintained during the first year of outpatient follow-up. This single case indicates the possible therapeutic efficacy of rTMS in narcoleptic patients with a favorable safety and tolerability.
2. Case presentation
A 14-year-old Chinese girl came to the hospital and complained of EDS, cataplexy and hypnagogic hallucinations for 5 years, but without any evidence of sleep paralysis. Her sleep attacks could possibly occur when she was studying, eating, standing, and walking, even without drastic mood swing. The moment she fell asleep and woke up, she often felt drowsy and could hear voices like mosquitoes buzzing. Her daytime sleepiness usually abated following a nap. Before admission, she had merely tried some traditional Chinese medicine, but quitted soon due to no improvement in symptoms.
She denied any central nervous system infections, brain trauma, and epilepsy in the past. She had no history of drug dependence, poisoning or long-term medication. Her menarche occurred when she was 12 years old, but her menstrual cycle was irregular, the interval ranged from 30 to 90 days, and her period was usually longer than 7 days. Her younger sister was generally healthy and did not experience similar symptoms.
Her physical examination was normal, with any positive neurological findings. Her weight was 70 kg, height was 160 cm, and body mass index was 27.3. In the last 2 years, her weight fluctuated between 70 and 75 kg. The hematological tests indicated mild hypertriglyceridemia, while serum levels of cortisol, adrenocorticotropic hormone, thyroid, and sexual hormones were all normal. Gynecological ultrasonography indicated polycystic ovaries, which may account for her menstrual problems. The electroencephalogram and brain magnetic resonance imaging examinations were normal. The polysomnography examination excluded obstructive sleep apnea and restless leg movement. Because of limited laboratory conditions, we could not perform HLA-DQB1×0602 test and cerebrospinal fluid hypocretin examination. As lack of evidence from multiple sleep latency tests, we primarily diagnosed the patient with narcolepsy-like symptoms.
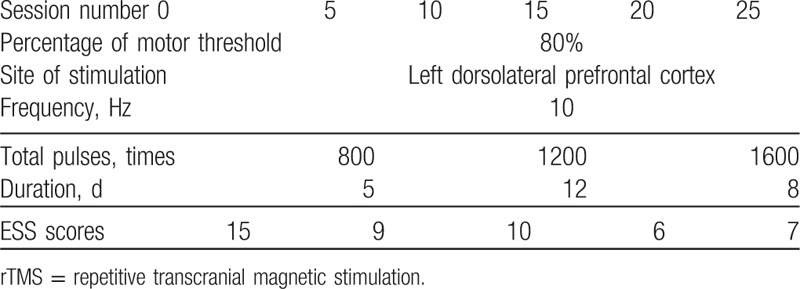
This patient was right-handed as identified by her dominant writing hand. After excluding potential contraindications, we delivered non-neuronavigating rTMS with a figure-8 coil (Magstim rapid stimulator, Magstim, Sheffield, UK, 10 Hz, 80% resting motor threshold) over the left DLPFC of this young girl for 5 sessions a week and 5 consecutive weeks, 25 sessions in total (Table 1). The resting motor threshold was identified as the lowest magnetic intensity over the motor cortex to elicit a finger movement for at least 5 times out of 10 stimuli. Stimulation was initiated with 20 trains of 4 seconds on and 12 seconds off, 800 daily pulses in the first 5 sessions in total, followed by 1200 daily pulses (30 trains) in the next 12 sessions, and ended with 1600 pulses (40 trains) in the last 8 sessions. No any other drug that can affect cortical excitability was allowed during the treatment. After every 5 sessions, her somnolence was evaluated by ESS, a scale widely used to measure daytime sleepiness.[17] Notably, her ESS score decreased from 15 at baseline to 7 at the end of stimulation, indicating favorable improvement in daytime sleepiness. Meanwhile, this patient reported her hypnagogic hallucinations also significantly alleviated. To continue her treatment, venlafaxine (75 mg/d), an antidepressant of the serotonin-norepinephrine reuptake inhibitors, was then administered after her discharge from the hospital. During the follow-up in the next year, she reported her remission in EDS and hypnagogic hallucinations maintained, without episodes of cataplexy.
Table 1.
rTMS parameters and the Epworth sleep scale (ESS) scores during the treatment.
This case study was approved by the Institute Ethical Committee of The First Affiliated Hospital, Zhejiang University School of Medicine. Signed informed consent was taken from the guardian of our patient.
3. Discussion
To the best of our knowledge, this is the first case report that high-frequency rTMS over the left DLPFC is possibly effective for narcolepsy-like symptoms. In this case, the patient experienced 25 sessions of 10 Hz magnetic stimulation with its intensity at 80% resting motor threshold. Throughout the treatment, this young patient was well-tolerate to our stimulation settings and showed apparent improvement in her daytime sleepiness, hypnagogic hallucinations, and cataplexy. Meanwhile, no rTMS-related adverse effects were observed during the treatment.
In our patient, the left DLPFC was selected as the stimulating target of rTMS. This brain area participates in regulating emotion and executive functions, such as working memory, attention, planning, organization, response inhibition.[18] Indeed, narcoleptic patients also have problems in attention, emotion, and memory. A recent large-scale epidemiological study demonstrated high burden of comorbid mental illnesses in narcoleptic patients (e.g., mood disorder and anxiety disorder).[19] Besides, the DLPFC region is one of the most popular targets of rTMS in various neuropsychiatric diseases, and magnetic stimulation over this area seems to be safe and tolerable.[12] The mechanism underlying rTMS treatment over left DLPFC is possibly by modulating the anterior cingulated cortex connectivity in the meso-cortico-limbic circuit.[20] As for narcoleptic patients, structural and functional abnormalities in the left DLPFC area have already been reported in previous studies.[8–10] Our case study indicates that rTMS treatment over left DLPFC may exhibit a sleep-modulating effect.
The primary medications for narcolepsy are modafinil, sodium oxybate, and venlafaxine. These drugs increase the concentration of monoaminergic neurotransmitters in brain to keep the mind awake.[11,15,16] However, before rTMS treatment, our patient and her guardian were afraid of the side effects and addiction of psychotropic medicines and refused to take any of the aforementioned drugs. rTMS was therefore recommended and initiated after signing informed consent. After the improvement of symptoms following rTMS treatment, our patient agreed to take low dose of venlafaxine (75 mg/d) monotherapy to continue her therapy. Her remission in symptoms maintained in the first year follow-up.
As a physical therapy, rTMS can either potentiate or decrease cortical excitability depending on the stimulation conditions. A recent study found that rTMS over the DLPFC decreased the alpha wave activity during the rapid-eye-movement sleep period in drug-resistant depressed patients, and this phenomenon was interpreted as an upregulation of cortical activity.[21] Meanwhile, rTMS can influence the efficiency of excitatory synaptic transmission, and neural plasticity, which further lead to long-term changes in cortical excitability.[22] Therefore, we speculate that in patients with narcolepsy, high-frequency rTMS over the left DLPFC may modulate sleep architecture by affecting the neurotransmission, changing the rhythm of neural activity, improving the regional perfusion, and eventually enhancing the cortical excitability.
To conclude, this single case report is descriptive and has not a few limitations, such as lack of controls, open-label design, and no standard stimulation parameters can be referred to. Of note, our patient did not receive any drug during rTMS treatment, and her narcolepsy-like symptoms remarkably improved after rTMS, which was also supported by the ESS evaluation. Given the long course of her disease and low probability to alleviate spontaneously, it was likely that the remission of our patient was associated with the treatment effect of rTMS. Another limitation is that no polysomnography examination was performed after rTMS stimulation to confirm the alteration in a narcolepsy-related sleep pattern. However, it was still indicative that high frequency over the left DLPFC might be an alternative choice for symptom control in narcoleptic patients with cataplexy. Future studies with a large sample and refined design are warranted to verify this topic.
Acknowledgments
The authors acknowledge the patient and her guardian for their understanding. The authors also thank the National Key Basic Research Program (2016YFC1307100), the National Science and Technology Program (2015BAI13B02), and the Key Research Project of Zhejiang Province (2015C03040) for their support.
Footnotes
Abbreviations: DLPFC = dorsolateral prefrontal cortex, EDS = excessive daytime sleepiness, ESS = Epworth sleep scale, rTMS = repetitive transcranial magnetic stimulation, SPECT = brain single photon emission computed tomography.
JL and MH contributed equally to this work.
This work was supported by the grants of the National Key Basic Research Program (2016YFC1307100), National Science and Technology Program (2015BAI13B02), and the Key Research Project of Zhejiang Province (2015C03040).
The authors have no conflicts of interest to disclose.
References
- [1].Burgess CR, Scammell TE. Narcolepsy: neural mechanisms of sleepiness and cataplexy. J Neurosci 2012;32:12305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].American Academy of Sleep Medicine. International classification of sleep disorders. Diagnostic and coding manual. 2nd edWestchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- [3].Taheri S, Zeitzer JM, Mignot E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu Rev Neurosci 2002;25:283–313. [DOI] [PubMed] [Google Scholar]
- [4].Partinen M, Kornum BR, Plazzi G, et al. Does autoreactivity have a role in narcolepsy? Lancet Neurol 2014;13:1072–3. [DOI] [PubMed] [Google Scholar]
- [5].Smith AJ, Jackson MW, Neufing P, et al. A functional autoantibody in narcolepsy. Lancet 2004;364:2122–4. [DOI] [PubMed] [Google Scholar]
- [6].Arango MT, Kivity S, Shoenfeld Y. Is narcolepsy a classical autoimmune disease? Pharmacol Res 2015;92:6–12. [DOI] [PubMed] [Google Scholar]
- [7].Salih F, Grosse P. Complete loss of F-waves during cataplectic attacks in patients with narcolepsy. Neurology 2014;83:2095–6. [DOI] [PubMed] [Google Scholar]
- [8].Joo EY, Jeon S, Lee M, et al. Analysis of cortical thickness in narcolepsy patients with cataplexy. Sleep 2011;34:1357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Joo EY, Hong SB, Tae WS, et al. Cerebral perfusion abnormality in narcolepsy with cataplexy. Neuroimage 2005;28:410–6. [DOI] [PubMed] [Google Scholar]
- [10].Hong SB. Neuroimaging of narcolepsy and Kleine-Levin Syndrome. Sleep Med Clin 2017;12:359–68. [DOI] [PubMed] [Google Scholar]
- [11].Thorpy MJ, Dauvilliers Y. Clinical and practical considerations in the pharmacologic management of narcolepsy. Sleep Med 2015;16:9–18. [DOI] [PubMed] [Google Scholar]
- [12].Lefaucheur JP, André-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014;125:2150–206. [DOI] [PubMed] [Google Scholar]
- [13].Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol 2003;2:145–56. [DOI] [PubMed] [Google Scholar]
- [14].Vijayakumari AA, Khan FR, Varma RP, et al. Can transcranial magnetic stimulation be used to evaluate patients with narcolepsy? Neurol Sci 2013;34:1411–20. [DOI] [PubMed] [Google Scholar]
- [15].Joo EY, Hong SB, Kim HJ, et al. The effect of modafinil on cortical excitability in patients with narcolepsy: a randomized, placebo-controlled, crossover study. Sleep Med 2010;11:862–9. [DOI] [PubMed] [Google Scholar]
- [16].Nardone R, Bergmann J, Lochner P. Modafinil reverses hypoexcitability of the motor cortex in narcoleptic patients: a TMS study. Sleep Med 2010;11:870–5. [DOI] [PubMed] [Google Scholar]
- [17].Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540–5. [DOI] [PubMed] [Google Scholar]
- [18].Feil J, Sheppard D, Fitzgerald PB, et al. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev 2010;35:248–75. [DOI] [PubMed] [Google Scholar]
- [19].Black J, Reaven NL, Funk SE, et al. Medical comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study. Sleep Med 2017;33:13–8. [DOI] [PubMed] [Google Scholar]
- [20].Tik M, Hoffmann A, Sladky R, et al. Towards understanding rTMS mechanism of action: stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage 2017;162:289–96. [DOI] [PubMed] [Google Scholar]
- [21].Pellicciari MC, Cordone S, Marzano C, et al. Dorsolateral prefrontal transcranial magnetic stimulation in patients with major depression locally affects alpha power of REM sleep. Front Hum Neurosci 2013;7:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Funke K, Benali A. Modulation of cortical inhibition by rTMS—findings obtained from animal models. J Physiol 2011;589(pt 18):4423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


