Figure 4. Titration of a11.2 IQ domain – derived peptides with apoCaM.
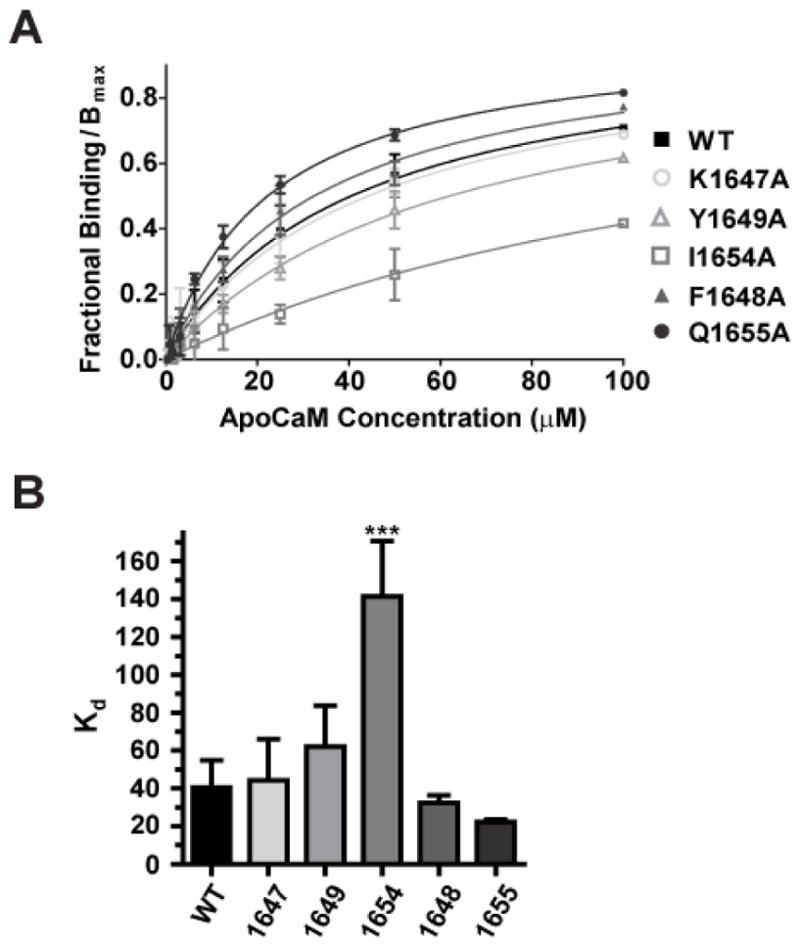
One μM fluorescein-labeled peptides spanning the α11.2 IQ domain (AAs 1644-1668) were incubated with serial dilutions of purified apoCaM. Changes in fluorescence polarization (FP) were measured to determine binding affinities of the individual IQ domain peptides. A) Shown are averages of fractional bindings normalized to Bmax against concentration of apoCaM. Error bars represent standard deviation of the fractional binding for each concentration. Binding curves were calculated by fitting the data to the equation Y = B*X/(Kd + X); B: maximal fractional binding value that would be reached at saturation as determined by extrapolation of the fitted curve. For fractional binding, the maximum and minimum polarization value for each titration curve was set to 1 and 0, respectively. B) Binding affinity Kd values were obtained from curve fitting. Only the I1654A mutant peptide showed reduced binding affinity to apoCaM relative to the WT α11.2 peptide, which was statistically significant (***p<0.001, Tukey post hoc test; N=3–6; see Table 1).
