Figure 9.
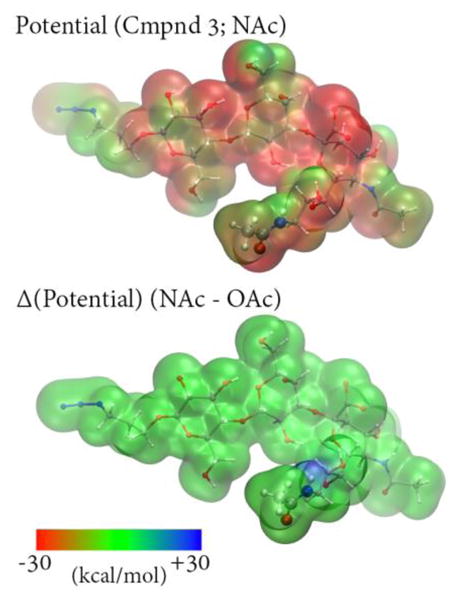
Top: Calculated electrostatic potential for a representative sialoside structure extracted from simulations of Neu5Ac9NAcα3Galβ4GlcβProN3 using the B3LYP/6-31G* level of theory60,61 and plotted on the ρ=0.002 density isosurface. Bottom: Electrostatic potential difference computed between Neu5Ac9NAcα3Galβ4GlcβProN3 and Neu5,9Ac2α3Galβ4GlcβProN3 plotted on the same isosurface; the O-acetylated potential was calculated by replacing NH with O in the N-acetylated structure. The difference between the potentials is very small, although NH group has a slightly positive potential relative to O.
