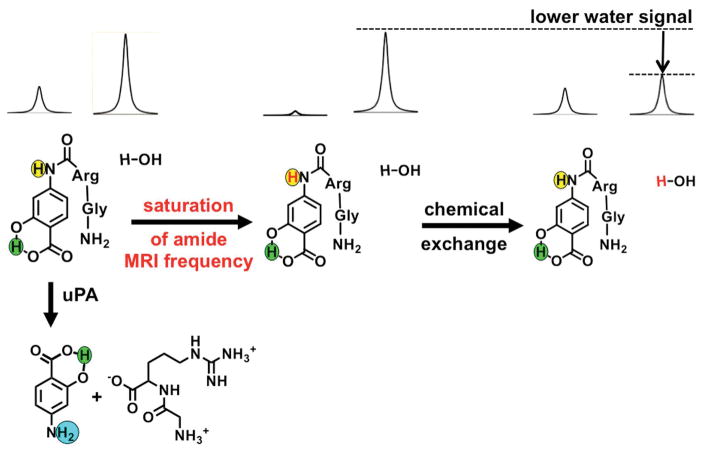
Figure 2.
The catalyCEST MRI mechanism. Top left: The aryl amide (highlighted in yellow) and the water proton have MR signals at distinct frequencies. Center: Selective saturation of the MR frequency of the amide proton causes a loss of the proton’s MR signal. Right: Chemical exchange of the amide and water protons transfers the saturation to water, leading to loss of the MR water signal. Bottom left: The CEST effect of the aryl amide proton is lost when uPA cleaves the peptidyl ligand to create an aryl amine (highlighted in cyan). The salicylic acid proton (highlighted in green) can generate a CEST effect, and is unresponsive to enzyme activity.