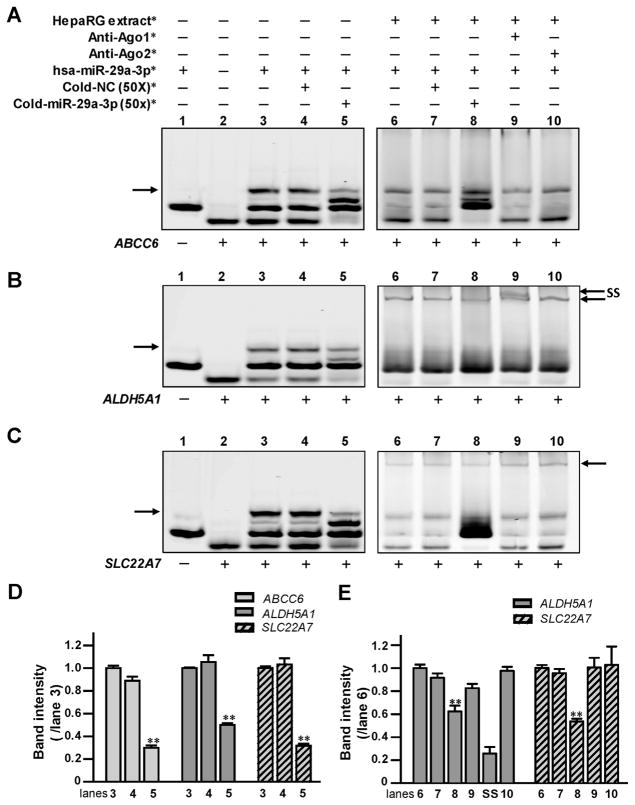
Fig. 2. The hsa-miR-29a-3p oligonucleotides directly interacted with (A) ABCC6, (B) ALDH5A1, and (C) SLC22A7 mRNA oligonucleotides in vitro.
*The reagents were used in RNA EMSA containing ABCC6,ALDH5A1, and SLC22A7 mRNA oligonucleotides, under the same experimental conditions. NC, nonspecific competitor. Lanes 1 and 2 indicated the mobility of each type of oligonucleotide; lane 3 indicated the mobility status of the miRNA:mRNA complex formed by the interaction of hsa-miR-29a-3p oligonucleotides with ABCC6, ALDH5A1, or SLC22A7 mRNA oligonucleotides; lanes 4 and 5 revealed the mobility shift status of miRNA:mRNA complex in the presence of excess unlabeled nonspecific competitors and specific competitors (hsa-miR-29a-3p). Lane 6 showed complexes formed using the cytoplasmic extracts from HepaRG cells incubated with hsa-miR-29a-3p oligonucleotides and ABCC6, ALDH5A1, and SLC22A7 mRNA oligonucleotides. Lanes 7 and 8 showed the mobility shift status of protein: miRNA:mRNA complexes in the presence of excess unlabeled nonspecific competitors and specific hsa-miR-29a-3p competitors. Lanes 9 and 10 indicated the mobility status of miRNA/mRNA/protein complexes with Ago1 or Ago2 antibodies. Arrows (left) indicate the oligonucleotide complexes in lane 3. Arrows (right) indicate the miRNA/mRNA/protein complexes formed by oligonucleotides and cytoplasmic proteins in lanes 6–10 in (B) and (C). SS indicates the supershift complex formed by the miRNA/mRNA/protein and antibody against Ago1 in lane 9 in (B). (D and E) Histogram corresponds to the relative densitometry quantification of the key miRNA/mRNA complexes or miRNA/mRNA/protein complexes observed in (A, B or C), compared to those in lane 3 or lane 6, respectively. **P <0.001.