Abstract
In healthy individuals, there is evidence that effective implementation of an emotion regulation strategy has beneficial effects on temporally proximal cognitive control task performance. This effect occurs because both of these processes rely heavily on the prefrontal cortex. Individuals with schizophrenia (SZ) have impairments in both emotion regulation and cognitive control that are driven by structural and functional abnormalities of the prefrontal cortex; however, it is unknown whether emotion regulation attempts fail to benefit subsequently performed cognitive control tasks in people with SZ. The present study examined whether attempts to increase or decrease negative emotion via reappraisal have differential effects on subsequent cognitive control in a sample of outpatients diagnosed with SZ (n = 30) and demographically matched healthy controls (CN: n = 29). Participants completed a combined emotion regulation and cognitive control task in which numerical Stroop trials were presented immediately after unpleasant or neutral images that were either increased via reappraisal, decreased via reappraisal, or passively viewed. The electroencephalogram was recorded while participants performed the reappraisal-Stroop task and event related potentials (ERPs) were used to index emotion regulation effectiveness (late positive potential: LPP) and cognitive control (sustained potential: SP). Both CN and SZ evidenced higher LPP amplitude for unpleasant than neutral stimuli consistent with robust neural response to unpleasant stimuli. Although CN demonstrated neurophysiological evidence of effective use of reappraisal to increase and decrease negative emotion, SZ only showed an effective ability to increase negative emotion via reappraisal. CN displayed enhanced cognitive control following increase trials and impaired cognitive control following decrease trials, as indicated by modulation of SP amplitude. In SZ, increase instructions impaired cognitive control and decrease instructions had no effect on cognitive control. Findings suggest that emotion regulation abnormalities may play an under-recognized role in general cognitive control deficits that occur in SZ.
Keywords: Emotion Regulation, Distancing, Psychosis, Affect, Event Related Potentials
Introduction
Emotion regulation refers to the processes used to increase or decrease the frequency, intensity, or duration of emotional response (Gross, 1998). Abnormalities in emotion regulation have been linked to risk for developing several psychiatric disorders (Aldao et al., 2010). James Gross’ process model is a useful conceptual framework for understanding the role of emotion regulation abnormalities in psychopathology (Gross, 1998; Gross et al., 2015). Gross proposes that the emotional response unfolds over a sequence of stages, and that strategies can be applied to control the emotion response at any one of these stages (Gross, 1998, 2002). Psychopathology may arise from abnormalities in identifying when an emotion regulation strategy should be attempted, selecting contextually appropriate strategies, or ineffectively implementing strategies after they are selected (Sheppes et al., 2015).
Relatively few studies have examined emotion regulation in schizophrenia (SZ), despite consistent evidence that the disorder is associated with heightened stress reactivity that predicts disease liability and severity of psychotic symptoms (Corcoran et al., 2003; Walker, Mittal & Tessner, 2008). Several studies examining self-reports made on emotion regulation questionnaires have found that SZ patients implement reappraisal (i.e., an adaptive strategy that entails reinterpreting the meaning of situations/stimuli so that they are less negative or more positive) less frequently and expressive suppression (i.e., an ineffective strategy that entails reducing the outward expression of emotion to reduce emotional experience) more frequently than healthy controls (Gross & John, 2003; Horan et al., 2013; Kimhy et al., 2012; Livingstone, Harper, & Gillanders, 2009; van der Meer, van't Wout, & Aleman, 2009). However other studies report no group differences in reappraisal or suppression on these measures (Badcock, Paulik, & Maybery, 2011; Henry et al., 2008; Perry, Henry, & Grisham, 2011; Rowland et al., 2013; van der Meer et al., 2014). Discrepant findings may reflect differences in demographics (e.g., sex, age), symptom characteristics, medication regimen, and stage of illness across studies. Despite these inconsistencies, reduced use of reappraisal and greater use of suppression is consistently associated with poor functional outcome and greater symptom severity in SZ (Butler, Gross, & Barnard, 2014; Henry et al., 2008; Kimhy et al., 2012; Perry et al., 2011; van der Meer et al., 2009).
Electrophysiological and neuroimaging studies provide more consistent evidence that SZ patients are less effective than healthy controls at implementing specific emotion regulation strategies. Two studies used an event related potential (ERP) paradigm modeled after Foti and Hajcak (2008) that presented participants with schizophrenia and healthy controls with unpleasant and neutral images that were preceded by an audio file that framed an upcoming image as either more or less arousing (Strauss et al., 2013; Horan et al., 2013). The late positive potential (LPP), a midline centoparietal ERP component that becomes evident at about 300ms and remains higher in amplitude for emotional than neutral stimuli during passive viewing, was examined to determine effectiveness of implementing the strategy. Results of both studies indicated that the audio files that framed the upcoming unpleasant image as less arousing significantly decreased (i.e., down-regulated) LPP amplitude to unpleasant stimuli in healthy controls (CN), but not people with SZ. Furthermore, the extent to which SZ patients could decrease the LPP using the cognitive change strategy predicted intensity of state and trait negative emotionality (Strauss et al., 2015). In another ERP study, Strauss et al. (2015) had healthy controls and individuals with SZ direct attention to either arousing or non-arousing aspects of an unpleasant scene to examine effectiveness at implementing a directed attention strategy. Results indicated that directing top-down attention toward non-arousing aspects of unpleasant scenes reduced LPP amplitude in healthy controls, but not SZ patients. Neuroimaging studies indicate that abnormalities observed on these ERP tasks may reflect prefrontal hypoactivation and dysfunctional cortico-limbic coupling (Morris et al., 2012; van der Meer et al., 2014). Thus, results from studies using diverse methodologies generally suggest that SZ patients are less effective than healthy controls at implementing emotion regulation strategies to control negative emotional response.
It is unclear whether abnormalities in implementing emotion regulation strategies also impact other processes relevant to the illness, such as general cognitive performance. The neural circuitry involved with emotion regulation typically includes dorsolateral and posterior prefrontal cortices, dorsal anterior cingulate cortex, and ventrolateral prefrontal cortex (Ochsner et al., 2012). This circuitry overlaps with that implicated in general cognitive control processes, which have been found to be impaired in SZ patients using neuroimaging and ERP methodologies (Markela-Lerenc et al., 2009; McNeely, West, Christensen, & Alain, 2003; Morris et al., 2012; van der Meer et al., 2014). Given that circuitry required for successful emotion regulation overlaps substantially with that required for cognitive control, one might expect emotion regulation ability to have a direct, causal effect on cognitive processing that occurs immediately after individuals attempt to regulate their emotions. Although this speculation seems plausible from a neurophysiological perspective, the nature of an expected interaction between emotion regulation and cognition is unclear- should increasing and decreasing negative emotion enhance or impair subsequent cognitive control?
To our knowledge, only one published study has examined the effects of emotion regulation on subsequent cognitive control processes in healthy individuals. Moser, Most, & Simons (2010) conducted an ERP study on healthy undergraduate students, which required participants to perform a cognitive control task (Stroop) immediately after increasing or decreasing negative emotion using reappraisal. On each trial, participants were first presented with a cue instructing them to “increase”, “decrease”, or maintain (i.e., passively view the image without engaging in emotion regulation) their emotions during the upcoming presentation of an image. An unpleasant or neutral stimulus was then presented during which time participants were supposed to apply the instruction presented in the cue. Immediately after the image, a numerical Stroop trial was presented where participants were asked to report the number of digits on the screen. Stroop trials were either congruent (e.g., 22) or incongruent (e.g., 222). The late positive potential (LPP) was used to index emotional reactivity and regulation effectiveness. The N450 and Sustained Potential were used to evaluate general cognitive control. The N450 is a frontocentral component that exhibits a phasic negativity around 400–500ms after Stroop onset, and reverses polarity over the lateral frontal regions (Lansbergen, van Hell, & Kenemens, 2007; West, 2003). The sustained potential (SP; sometimes called the conflict SP) is also involved in conflict evaluation and onsets around 600 ms after stimulus onset. It is marked by greater centroparietal positivity on incongruent trials, as compared to congruent trials (Lansbergen et al, 2007; West, 2003). Using the reappraisal-Stroop paradigm, Moser et al. (2010) found evidence for successful emotion regulation, as indicated by greater LPP amplitude for increase relative to unpleasant passive viewing, and significantly lower LPP amplitude in the decrease relative to unpleasant passive viewing condition. However, more importantly, there was a significant effect of emotion regulation on cognitive control performance. Specifically, relative to the unpleasant passive viewing condition, the increase condition enhanced Stroop performance as indicated by significantly faster reaction times (RT) on the incongruent trials and an increase in amplitude of the Sustained Potential for incongruent compared to congruent trials. There was no significant effect of the decrease condition on RT, N450, or the sustained potential. Thus, effectively implementing emotion regulation strategies to increase negative emotion may improve subsequent cognitive control, which may be sustained for a brief interval after an emotion regulation attempt occurs.
Whether emotion regulation attempts have beneficial or detrimental effects on subsequent cognitive control performance in SZ is currently unknown. There is consistent evidence for abnormal prefrontal structure and function in schizophrenia (Barch et al., 2001; Pantelis et al., 1997; Raine et al., 1992), cognitive control deficits (see Barch & Sheffield, 2014 for review), and preliminary evidence for a neurophysiological emotion regulation abnormality (Horan et al., 2013; Morris et al., 2012; Strauss et al., 2015; Strauss et al., 2013; van der Meer et al., 2014). Given perturbation to the prefrontal-limbic circuits in schizophrenia, one might not expect a pattern of enhanced subsequent cognitive control following attempts to increase negative affect via reappraisal. Rather, dysfunction within prefrontal and limbic structures, as well as their disrupted functional connectivity, may cause attempts to increase or decrease negative affect via reappraisal to impair subsequent cognitive control.
The present study sought to investigate whether increasing or decreasing negative emotion via reappraisal has a detrimental impact on concurrently performed cognitive control processes. In order to investigate the effects of reappraisal on cognitive control, the ERP paradigm developed by Moser, Most, & Simons (2010) was administered to chronic SZ patients and healthy controls. The following hypotheses were made: 1) SZ and controls would display greater amplitude of the LPP for the unpleasant passive viewing than neutral condition; 2) controls would evidence successful emotion regulation as indicated by lower LPP amplitude in the decrease condition compared to unpleasant passive viewing condition and higher LPP amplitude in the increase condition compared to the unpleasant passive viewing condition. However, individuals with SZ would display abnormalities in emotion regulation as indicated by no differences in amplitude of the LPP in the increase, decrease, and unpleasant passive viewing conditions; 3) Basic Stroop performance would be impaired in SZ for trials following neutral stimuli, as indicated by no significant differences in SP amplitude between congruent and incongruent conditions; 4) In line with Moser, Most, & Simons (2010), controls were expected to display enhanced cognitive control following the increase trials, as indicated by higher SP amplitude for incongruent than congruent trials; however, this effect was not predicted to occur in SZ patients who were expected to show no difference in SP amplitude between congruent and incongruent trials in the increase condition; 5) In SZ, poorer performance on both the emotion regulation and cognitive control portions of the task were expected to be associated with higher trait negative affect, poorer functional outcome, and greater symptom severity.
Method
Participants
Data was collected from two participant groups: 1) 32 individuals with DSM-IV-TR (APA, 2000) diagnoses of schizophrenia (n = 25) or schizoaffective disorder (n = 7) (SZ), and 2) 30 psychologically and neurologically healthy control participants (CN). Two SZ and one CN were excluded due to excessively noisy EEG data (> 50% of trials rejected due to EMG artifacts). Groups did not significantly differ on age, ethnicity, sex, or parental education; however, SZ had lower personal education than CN (see Table 1).
Table 1.
Demographic and Clinical Characteristics of the Sample
| SZ (n = 30) | CN (n =29) | Test Statistic, p-value | |
|---|---|---|---|
| Age | 38.60(11.98) | 41.97(12.94) | F= 1.08, p= 0.30 |
| Participant Education | 12. 97(2.13) | 15.14(1.94) | F= 16.49, p<0.001 |
| Parental Education | 13.5(2.62) | 13.92(2.11) | F= 0.45, p= 0.50 |
| % Male | 60% | 62.1% | χ2= 0.03, p= 0.87 |
| Race % | χ2= 3.54, p= 0.62 | ||
| Caucasian | 73.3% | 79.3% | |
| African-American | 6.7% | 3.4% | |
| Hispanic | 6.7% | 10.3% | |
| Asian | 0% | 3.4% | |
| Native American | 3.3% | 0% | |
| Mixed-Race | 10% | 3.4% | |
| PANAS Negative Affect | 26.2(9.11) | 15.68(5.35) | F= 28.09, p < 0.001 |
| BNSS | |||
| Total | 22.65(18.16) | -- | -- |
| Motivation/Pleasure Dimension | 2.11(1.54) | -- | -- |
| Expressivity Dimension | 1.46(1.72) | -- | -- |
| BPRS | |||
| Positive Symptoms | 3.16(1.32) | -- | -- |
| Negative Symptoms | 2.11(1.20) | -- | -- |
| Disorganized Symptoms | 2.12(0.93) | -- | -- |
| Total | 47.00(10.46) | ||
| LOF | |||
| Social | 5.93(2.90) | -- | -- |
| Work | 1.90(2.69) | -- | -- |
| Total | 19.03(7.74) | -- | -- |
| Antipsychotic Medications | |||
| % Prescribed 1st Generation | 6.7% | -- | -- |
| % Prescribed 2nd Generation | 56.7% | -- | -- |
| % Prescribed 1st and 2nd Gen. | 3.3% | ||
| % Stably Unmedicated | 33.3% | -- | -- |
Note: SZ = Schizophrenia Patients; CN = Healthy Controls; PANAS = Positive and Negative Affect Scale; BNSS = Brief Negative Symptom Scale; BPRS = Brief Psychiatric Rating Scale; LOF = Level of Function Scale
Individuals with SZ were recruited from local community outpatient mental health centers and identified by their treating psychiatrist for inclusion in the study if they carried a primary diagnosis of DSM-IV schizophrenia or schizoaffective disorder (American Psychiatric Association, 2000). Clinical diagnosis was confirmed using the Structured Clinical Interview for DSM-IV-TR (SCID: First et al., 2002), which was informed by consultation with the patient’s treating psychiatrist, other program staff, and review of medical records. CN participants were recruited from the local community using posted flyers, newspapers advertisements, and electronic advertisements. CN had no current Axis I or II DSM-IV diagnoses as established by the SCID-I and SCID-II (First et al., 2002; Pfol et al., 1997), no family history of psychosis, and none were taking psychotropic medications. All participants were free from lifetime neurological disease and substance use disorders within the last 6 months. All participants received monetary compensation for their participation. All participants provided written informed consent for a protocol approved by the Binghamton University Institutional Review Board (protocol 2298-13: Motivated Attention and Avolition in Individuals with Schizophrenia).
Procedures
Prior to completing the behavioral and ERP tasks, examiners who were trained to reliability standards, conducted a structured diagnostic interview with participants to complete the SCID-I and SCID-II and determine Axis I and II diagnoses. A clinical interview was also completed to assess symptom severity over the past week for SZ patients, after which the following measures were rated: Brief Psychiatric Rating Scale (BPRS) (Overall & Gorham, 1962), Brief Negative Symptom Scale (BNSS) (Kirkpatrick et al., 2011), and Level of Function Scale (LOF) (Hawk, 1975).
ERP Emotion Regulation and Cognitive Control Task
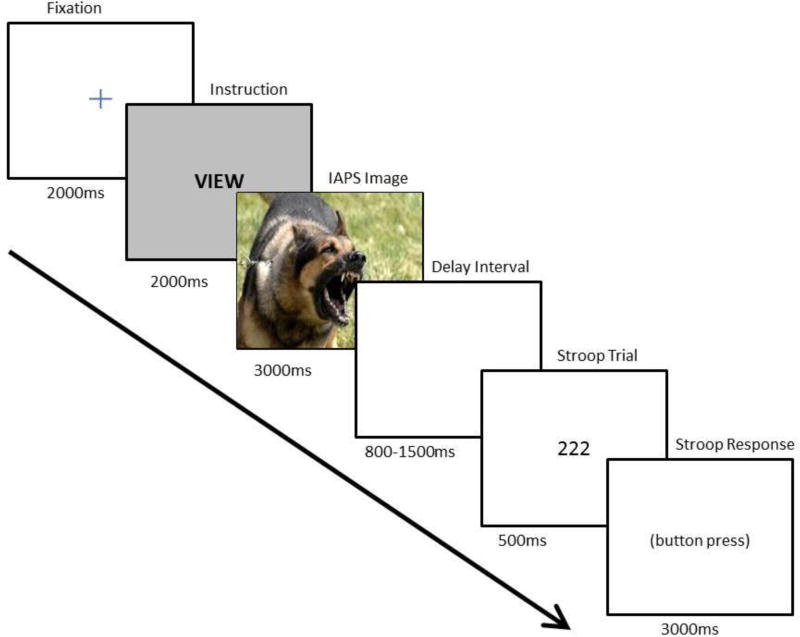
Participants completed an emotion regulation and cognitive control task modeled after Moser, Most, & Simons, 2010 while the electroencephalogram (EEG) was recorded. The task interleaves emotion regulation and Stroop presentations into a single trial to evaluate the effects of decrease and increase reappraisal instructions on subsequent cognitive control processes. A sample trial diagram is provided in Figure 1.
Figure 1. Sample Trial Sequence.
Note. Participants performed a numerical Stroop trial immediately after completing an instructed emotion regulation task that required passively viewing unpleasant or neutral stimuli or increasing or decreasing negative emotion using a distancing reappraisal strategy. Behavioral and ERP data were recorded.
On each trial, a fixation cross was presented in the center of a black screen for 2000 ms to orient attention to the upcoming display. Next, a written instruction was provided (“Increase”, “Decrease”, “View”, or “Watch”) for 2000 ms. Using a procedure similar to Thiruchselvam, Blechert, Sheppes, Rydstrom, & Gross (2011), in order to help orient participants to the different trial types, the cue screen was color-coded according to the type of instruction. The colors for each trial type were as follows: gray for VIEW, black for WATCH, blue for INCREASE, and green for DECREASE. Immediately following the instruction cue, an unpleasant or neutral image from the International Affective Picture System (IAPS) was presented for 3000 ms. After an interstimulus interval randomly selected to be between 800 and 1500 ms, a numerical Stroop trial was displayed on the monitor for 500 ms. Once the numerical Stroop presentation disappeared, participants were allowed to provide a behavioral response, where they indicated the number of digits on the screen as quickly as possible using a video game controller.
Prior to task initiation, the experimenter explained basic experimental procedures and gave specific directions to participants about emotion regulation and the strategy of distancing (detailed below). Participants were then guided through a combined training and practice session, which was modeled after Thiruchselvam et al., (2011). Training involved systematic explanation of the construct of reappraisal, providing examples of using increase and decrease to alter negative emotion to IAPS stimuli, and having participants verbally describe the thought they implemented to alter their emotion using reappraisal. During the practice session, participants completed a series of separate emotion regulation and Stroop practice trials, and then a combined regulation-Stroop practice that directly emulated the task.
After training and practice, EEG electrodes were attached to the participant. The experimental session included 120 trials, divided into 2 blocks of 60 trials each with mandatory 30 second breaks after each set of 30 stimuli. There were four types of emotion regulation trials (increase, decrease, watch, view) and 2 types of Stroop trials (congruent, incongruent). In the two reappraisal conditions, participants were instructed to increase or decrease their emotional response to unpleasant images using the emotion regulation strategy of “distancing”. Distancing involves thinking about the personal relevance of each image as it is presented. When asked to increase their emotional response, participants were directed to increase their subjective closeness to the stimuli by imagining themselves or a loved one as the central focus of the depicted situation or as present in the image. When asked to decrease emotional response, participants were directed to increase their objective distance from the image, viewing the events in the image from a detached, third-person perspective. When asked to “view” (unpleasant) or “watch” (neutral), participants were directed to attend to the image and allow their thoughts and feelings to arise naturally. Although the view and watch trials were identical in instruction, separate cues were used for the two conditions in an attempt to maintain the same level of anticipatory knowledge participants had about the subsequent images across all four types of emotion regulation trials.
Participants were asked to complete the instruction only after picture onset and to keep their eyes open and on the screen the entire time. Each of the 2 blocks contained 15 view and 15 watch trials, as well as either 30 increase or 30 decrease trials, for a total of 60 trials per block. The blocking procedure was implemented to ensure that no block contains trials from both strategies, in an effort to decrease the likelihood of confusing which strategy should be implemented on a given trial. This procedure is consistent with Moser et al. (2009). For each instruction across both blocks, Stroop trials were counterbalanced such that half of the Stroop trials were congruent and half were incongruent. Block order was counter balanced and within each block, the order of the 60 trials was randomized for each participant. Mandatory 30 second breaks were provided between blocks.
ERP Task Stimuli
Experimental session stimuli consisted of 90 unpleasant and 30 neutral color images taken from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008). The unpleasant images included disgust and threat (human and animal). Unpleasant and neutral stimuli to be used significantly differed in valence (Unpleasant M=2.6, SD=0.53; Neutral M=5.3, SD=0.33; F(1, 118)=644.2, p<.001) and arousal (Unpleasant M = 5.6, SD=0.72; Neutral 3.5, SD=0.76, (F(1,118)=196.9, p<.001) according to combined male/female IAPS norms. Unpleasant stimuli were randomly assigned to conditions for each participant, ensuring that all stimuli had an equal probability of occurring in the unpleasant passive viewing, increase, and decrease conditions. Stimuli were displayed across the entirety of the screen (19′′ monitor, 1280 × 1024 resolution, 60 Hz refresh rate) at a viewing distance of approximately 70 cm and a visual angle subtending 32.6° × 21.0°. The practice and experimental stimuli did not overlap.
Numeric Stroop stimuli consisted of the numbers 1, 2, and 3 appearing one, two, or three times in a horizontal row on each trial, producing trails that are congruent (e.g. 333) and incongruent (e.g. 33). Stroop stimuli were displayed in the center of the screen within the foveal range at a visual angle subtending 3.5° × 1.6°.
EEG Recording, Data Reduction, and Analysis
EEG Recording
The EEG was recorded from 64 Ag/AgCl electrodes mounted in an elastic cap from manufactured by BrainVision (ActiCap model). The signals were recorded online using a right mastoid reference electrode and re-referenced offline to the average of the left and right mastoid. The horizontal electrooculogram (EOG) was used to measure horizontal eye movements and was recorded as the voltage between electrodes placed lateral to the external canthi. The vertical EOG was used to detect eyeblinks and vertical eye movements and was recorded from electrodes above and beneath the left eye. All electrode impedances were maintained below 15KΩ. The EEG and EOG were amplified by a BrainVision actiCHamp amplifier with a gain of 5,000, a bandpass filter of 0.05–100 Hz, and a 60-Hz notch filter. The amplified signals were digitized at 500 Hz and averaged offline.
EEG Data Reduction
All signal processing and analysis procedures were performed in Matlab using EEGLAB and the ERPLAB toolbox (Lopez-Calderon & Luck, 2014). Data preprocessing included the removal of large muscle artifacts or extreme offsets (identified by visual inspection). Independent component analysis (ICA) was conducted on the continuous data to identify and remove eyeblink activity.
LPP Measurement Procedures
ERPs were constructed by separately averaging trials from the four conditions of interest. The ICA-corrected EEG data was divided into epochs that began 200 ms prior to the onset of the stimulus and continued for 3000ms, and was baseline corrected using a 200 ms pre-stimulus period. The LPP was calculated as the average amplitude at electrodes Cz, CPz, and Pz from 300ms to 3000ms. Measurement procedures are consistent with prior work in this area and this task (e.g. Hajcak, Moser, & Simons, 2006; Horan et al., 2013; Strauss et al., 2013).
Sustained Potential Measurement Procedures
For the Stroop locked ERP analyses, separate averages were conducted for a 2 (incongruent, congruent) × 4 (increase, decrease, unpleasant passive viewing, neutral) matrix. A 200ms baseline correction was applied for the sustained potential, which was measured as the mean amplitude at electrodes P3, Pz, and P4 in the 750- to 900-msec post-Stroop-onset window. These procedures are similar to procedures that have been used previously (e.g. McNeely et al., 2003; Moser et al., 2010)
Results
Preliminary Analyses
A preliminary one-way ANOVA was conducted on experimenter ratings of reappraisal ability from the training phase. SZ were rated as producing significantly less effective reappraisals than CN, F(1, 57) = 4.25, p < 0.05. All subsequent analyses were conducted with and without participants who were rated by experimenters as having poor ability to generate reappraisals during the training phase. Removing the participants who were rated as being ineffective at reappraisal did not change the pattern of results observed and all significant effects remained significant. Thus, all analyses reported below include all participants regardless of experimenter rating of reappraisal ability.
Emotional Reactivity and Emotion Regulation
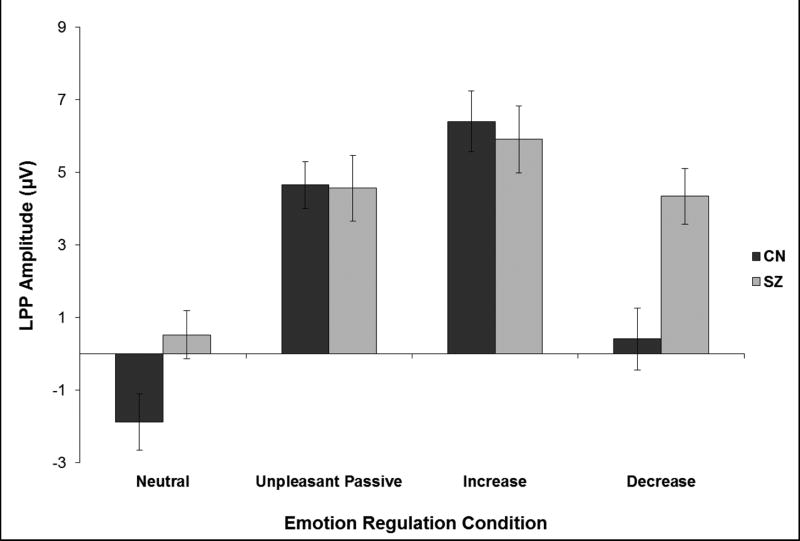
To test the hypothesis that CN and SZ would evidence a different pattern of LPP amplitude across the four emotion regulation conditions, a 2 Group (SZ, CN) × 4 Emotion Regulation condition (Neutral, Unpleasant Passive Viewing, Increase, Decrease) mixed model ANOVA was conducted (see Table 2)1. A significant interaction was observed, which was followed-up by post hoc one-way ANOVAs and within-group paired-samples t-tests. Results indicated that CN demonstrate greater LPP amplitude for unpleasant than neutral stimuli, as well as lower LPP amplitude for decrease than unpleasant passive viewing and higher amplitude for increase than unpleasant passive viewing. These findings suggest that CN can use increase and decrease instructions to regulate negative emotion effectively. Similarly, SZ showed greater LPP amplitude for unpleasant passive viewing than neutral trials and greater LPP amplitude for increase than unpleasant passive viewing trials; These findings suggest that SZ can use increase instructions to effectively up-regulate negative emotion. However, SZ did not display a difference between unpleasant passive viewing and decrease trials, indicating a deficit in down-regulating negative emotion via reappraisal (see Figures 2 and 3).
Table 2.
Omnibus ANOVA and Post Hoc Results for Late Positive Potential Emotional Reactivity and Regulation Conditions
| Test-Statistic | P-Value | Cohen’s d | |
|---|---|---|---|
| Omnibus ANOVA | |||
|
| |||
| Group | F (1,57) = 2.14 | 0.15 | 0.40 |
| Emotion Regulation | F (3, 171) = 81.14 | <0.001 | 2.39 |
| Group × Emotion Regulation | F (3, 171) = 10.13 | <0.001 | 0.84 |
|
| |||
| Post Hoc One-Way ANOVAs | |||
|
| |||
| Unpleasant Passive Viewing | F (1, 58) = 0.01 | 0.93 | 0.03 |
| Increase | F (1, 58) = 0.16 | 0.69 | 0.11 |
| Decrease | F (1, 58) = 11.73 | <0.01 | 0.91 |
| Neutral | F (1, 58) = 5.51 | 0.02 | 0.62 |
|
| |||
| Post Hoc within-Group Paired-Samples T-tests | |||
|
| |||
| Control | |||
| Neutral vs. Unpleasant Passive | t(28) = 13.27 | <0.001 | 5.02 |
| Neutral vs. Increase | t(28) = 12.72 | <0.001 | 4.81 |
| Neutral vs. Decrease | t(28) = 7.37 | <0.01 | 2.79 |
| Unpleasant Passive vs. Increase | t(28) = 3.26 | <0.001 | 1.23 |
| Unpleasant Passive vs. Decrease | t(28) = −3.26 | <0.001 | −1.23 |
| Decrease vs. Increase | t(28) = 8.85 | <0.001 | 3.35 |
| Schizophrenia | |||
| Neutral vs. Unpleasant Passive | t(29) = 5.20 | <0.001 | 1.93 |
| Neutral vs. Increase | t(29) = 6.14 | <0.001 | 2.28 |
| Neutral vs. Decrease | t(29) = 4.99 | <0.001 | 1.85 |
| Unpleasant Passive vs. Increase | t(29) = 1.86 | 0.07 | 0.69 |
| Unpleasant Passive vs. Decrease | t(29) = 0.41 | 0.69 | 0.15 |
| Decrease vs. Increase | t(29) = 3.02 | <0.01 | 1.12 |
Note. Pot hoc one-way ANOVAs tested the effect of Group on the indicated contrast.
Figure 2. LPP Mean Amplitude by Group and Emotion Regulation Condition.
Note: Results indicated a significant Group × Emotion Regulation interaction (p<0.01). The Figure demonstrates that both groups had greater LPP amplitude for unpleasant passive viewing than neutral conditions. Both groups also displayed greater LPP amplitude for the increase than unpleasant passive viewing condition; however, only the CN group showed significantly lower LPP amplitude for decrease than unpleasant passive viewing. These findings suggest that reactivity to unpleasant stimuli and the ability to increase negative emotion via reappraisal are intact in SZ; however, SZ display a deficit in decreasing negative emotion via reappraisal. LPP = Late Positive Potential; SZ = Schizophrenia patients; CN = Healthy controls; Error bars reflect standard error; LPP values reflect mean amplitude.
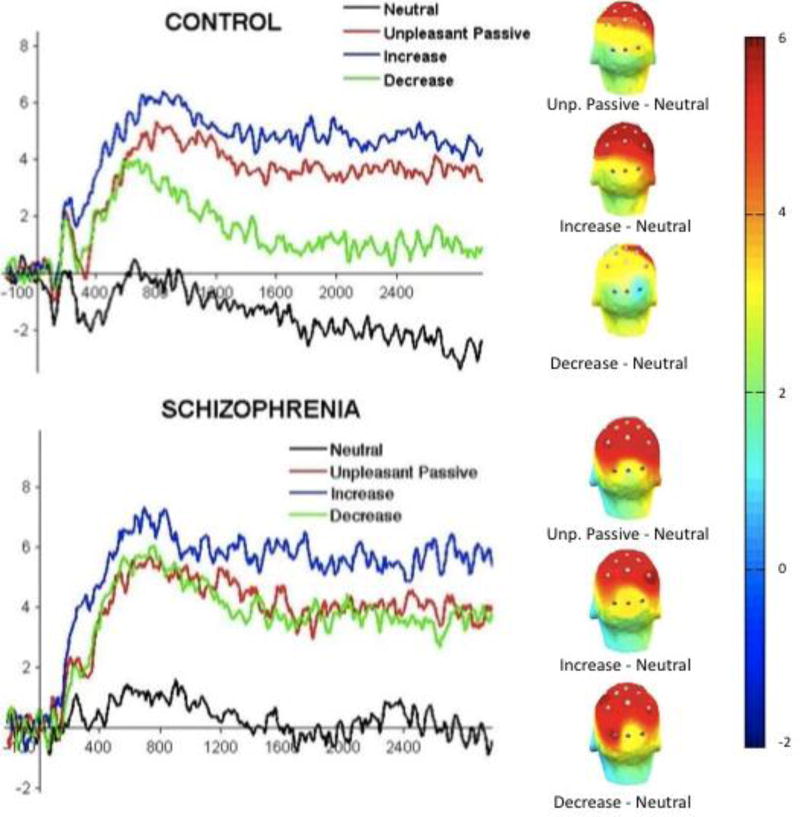
Figure 3. LPP Grand Average Waveforms by Group and Emotion Regulation Condition.
Note: Waveforms illustrate that CN were adept at decreasing and increasing negative emotion via reappraisal; SZ were adept at increasing, but not decreasing negative emotion via reappraisal. Scalp maps demonstrate that SZ and CN displayed the LPP at the expected centroparietal midline electrodes. LPP = Late Positive Potential; The LPP is evaluated at 300 – 3000 ms post-stimulus onset. Grand average waveforms depict time-series data for the means displayed in Figure 2.
The Effect of Emotion Regulation on Stroop Performance
Behavioral Analyses
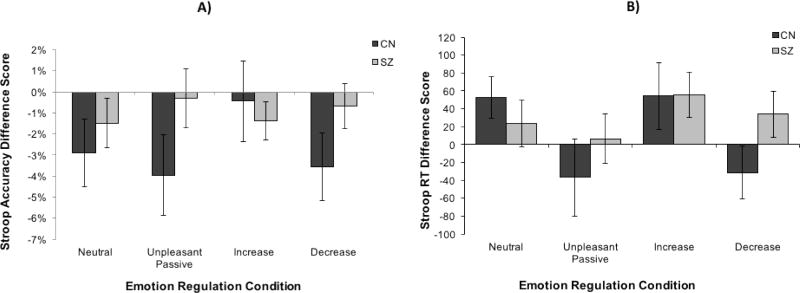
The differential effect of emotion regulation instruction on Stroop reaction time was examined using two separate 2 Group (CN, SZ) × 4 Emotion Regulation condition (Neutral, Unpleasant Passive Viewing, Increase, Decrease) mixed model ANOVAs with Stroop reaction time and accuracy interference scores (Incongruent trials – Congruent trials) as dependent variables. All main effects and interactions were nonsignificant (see Table 3 and Figure 4).
Table 3.
Omnibus ANOVA and Post Hoc Results Examining the Effect of Emotion Regulation on Stroop Performance
| F-Value | P-Value | Cohen’s d | |
|---|---|---|---|
| Stroop RT Interference Scores | |||
|
| |||
| Group | F(1, 57) = 0.66 | 0.42 | 0.20 |
| Emotion Regulation | F (2.02, 115.4)= 2.57 | 0.06 | 0.43 |
| Group × Emotion Regulation | F 2.02, 115.4) = 1.10 | 0.34 | 0.28 |
|
| |||
| Stroop Accuracy Interference Scores | |||
|
| |||
| Group | F (1, 57) = 1.17 | 0.28 | 0.29 |
| Emotion Regulation | F (2.63, 149.7) = 0.57 | 0.62 | 0.20 |
| Group × Emotion Regulation | F (2.63, 149.7) = 1.52 | 0.21 | 0.33 |
|
| |||
| Sustained Potential | |||
|
| |||
| Group | F (1, 57) = 3.86 | 0.054 | 0.063 |
| Emotion Regulation | F (3, 171) = 1.52 | 0.21 | 0.026 |
| Group × Emotion Regulation | F (3, 171) = 4.65 | <0.01 | 0.75 |
|
| |||
| Sustained Potential Post Hoc One-Way ANOVAs | |||
|
| |||
| Neutral Interference | F (1, 58) = 0.54 | 0.47 | 0.20 |
| Unpleasant Passive Interference | F (1, 58) = 3.27 | 0.076 | 0.48 |
| Increase Interference | F (1, 58) = 8.62 | <0.01 | 0.78 |
| Decrease Interference | F (1, 58) = 4.32 | <0.05 | 0.55 |
Note. Pot hoc one-way ANOVAs tested the effect of Group on the indicated contrast.
Figure 4. Stroop Behavioral Data by Group, Emotion Regulation Condition, and Stroop Condition.
Note: SZ = Panel A represents Stroop accuracy difference scores between congruent and incongruent Stroop trials by emotion regulation condition. A negative accuracy difference score indicates greater accuracy on congruent trials than incongruent trials. Panel B represents Stroop reaction time interference scores for congruent and incongruent Stroop trials by emotion regulation condition. A positive RT interference score indicates longer reaction time on incongruent trials than congruent trials. Error bars reflect standard error. The Group × Emotion regulation condition was nonsignificant for accuracy and RT. Schizophrenia patients; CN = Healthy controls. Behavioral dependent variables may have been less sensitive than ERP dependent variables.
Sustained Potential ERP Analyses
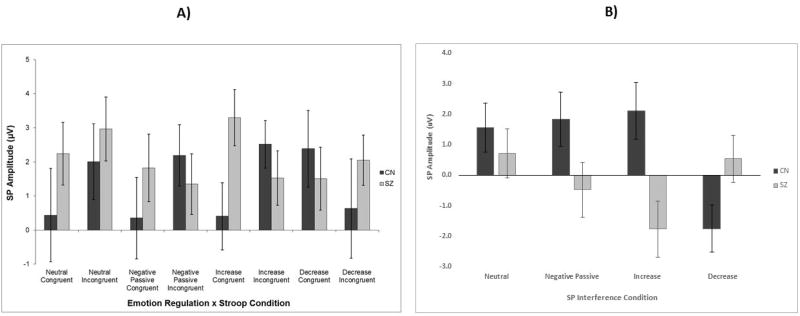
To investigate the effect of emotion regulation instruction on sustained potential amplitude, a 2 Group (CN, SZ) × 4 Emotion Regulation condition (Neutral, Unpleasant Passive, Increase, Decrease) mixed model ANOVA was conducted on SP interference scores (incongruent – congruent)2. There were no significant main effects. However, the Group × Emotion Regulation condition interaction was significant (Figure 5 and Table 3). Post hoc one-way ANOVAs indicated that the SP interference score was significantly higher in CN than SZ for the increase condition and significantly lower in SZ than CN for the decrease condition. Within-Group Paired Samples t-tests further clarified the directionality of the one-way ANOVA interference score results. CN had significantly greater SP amplitude for incongruent than congruent trials in unpleasant passive viewing, increase, and neutral conditions, and significantly higher SP amplitude for congruent than incongruent stimuli in decrease trials. In contrast, SZ failed to evidence differences in SP amplitude between congruent and incongruent trials on unpleasant passive viewing, neutral, or decrease conditions; however, they had significantly higher SP amplitude for congruent than incongruent trials during the increase condition. These findings indicate that increase instructions enhance cognitive control in CN, but impair it in SZ. In contrast, decrease instructions impair cognitive control in CN, but not in SZ (see Tables 3,4,5 and Figures 5,6,7).
Figure 5. SP Amplitude by Group, Emotion Regulation Condition, and Stroop Condition.
Note: Panel A shows mean SP amplitude per Stroop and Emotion Regulation condition in each group; Panel B shows SP interference scores (incongruent-congruent) for each emotion regulation condition. SZ = Schizophrenia patients; CN = Healthy controls; SP = Sustained Potential; Error bars reflect standard error; SP values reflect mean amplitude. There was a significant Group × Emotion regulation interaction (p<0.01) using SP interference scores as the dependent variable. As can be seen in the Figure, increasing negative emotion enhanced conflict detection in CN (positive difference scores); however, this was not true of SZ who showed a trend in the opposite direction of having conflict monitoring ability detrimentally affect by increasing negative emotion. In contrast, CN showed a negative SP interference score for the decrease condition, indicating that decrease instructions impair conflict monitoring; the effect of decrease instruct on conflict monitoring was not observed in SZ, potentially because they could not decrease effectively.
Table 4.
Post Hoc Within-Group Paired Sample T-Tests Comparing Congruent and Incongruent Conditions for the Sustained Potential
| Contrast | t-statistic | p-value | Cohen’s d | |
|---|---|---|---|---|
| CN | Neutral Incongruent vs Congruent | t(28) = 2.6 | <0.02 | 0.98 |
| Unp. Pass. Incongruent vs Congruent | t(28) = 2.1 | <0.05 | 0.79 | |
| Increase Incongruent vs. Congruent | t(28) = 3.3 | <0.01 | 1.25 | |
| Decrease Incongruent vs. Congruent | t(28) = −2.5 | <0.03 | −0.95 | |
|
| ||||
| SZ | Neutral Incongruent vs Congruent | t(29) = 0.8 | 0.46 | 0.30 |
| Unp. Pass. Incongruent vs Congruent | t(29) = −0.52 | 0.61 | −0.19 | |
| Increase Incongruent vs. Congruent | t(29) = −1.5 | 0.10 | −0.57 | |
| Decrease Incongruent vs. Congruent | t(29) = 0.7 | 0.52 | 0.26 | |
Note. CN = Healthy Control; SZ = Schizophrenia
Table 5.
Post-Hoc Within-Groups Paired Samples T-Tests of Sustained Potential Amplitude for Congruent and Incongruent Stroop Trials per Emotion regulation Condition
| Contrast | t-statistic | p-value | Cohen’s d | |||
|---|---|---|---|---|---|---|
| Congruent | ||||||
| CN | Neutral | Unpleasant Passive | t(28) = 0.11 | 0.91 | 0.04 | |
| Neutral | Increase | t(28) = 0.03 | 0.97 | 0.01 | ||
| Neutral | Decrease | t(28) = −2.84 | < 0.01 | −1.07 | ||
| Unpleasant Passive | Increase | t(28) = −0.06 | 0.95 | −0.02 | ||
| Unpleasant Passive | Decrease | t(28) = −2.98 | < 0.01 | −1.13 | ||
| Increase | Decrease | t(28) = −2.89 | < 0.01 | −1.09 | ||
|
| ||||||
| SZ | Neutral | Unpleasant Passive | t(29) =0.49 | 0.63 | 0.18 | |
| Neutral | Increase | t(29) = −0.92 | 0.37 | −0.34 | ||
| Neutral | Decrease | t(29) = 0.77 | 0.45 | 0.29 | ||
| Unpleasant Passive | Increase | t(29) = −1.37 | 0.18 | −0.51 | ||
| Unpleasant Passive | Decrease | t(29) = 0.31 | 0.76 | 0.12 | ||
| Increase | Decrease | t(29) = 1.58 | 0.12 | 0.59 | ||
|
| ||||||
| Incongruent | ||||||
|
| ||||||
| CN | Neutral | Unpleasant Passive | t(28) = −0.22 | 0.83 | −0.08 | |
| Neutral | Increase | t(28) = −0.58 | 0.57 | −0.21 | ||
| Neutral | Decrease | t(28) = 1.81 | 0.08 | 0.68 | ||
| Unpleasant Passive | Increase | t(28) = −0.54 | 0.60 | −0.20 | ||
| Unpleasant Passive | Decrease | t(28) = 1.40 | 0.17 | 0.53 | ||
| Increase | Decrease | t(28) = 1.54 | 0.14 | 0.58 | ||
|
| ||||||
| SZ | Neutral | Unpleasant Passive | t(29) = 2.47 | 0.02 | 0.92 | |
| Neutral | Increase | t(29) = 1.63 | 0.11 | 0.61 | ||
| Neutral | Decrease | t(29) = 1.10 | 0.28 | 0.41 | ||
| Unpleasant Passive | Increase | t(29) = −0.24 | 0.81 | −0.09 | ||
| Unpleasant Passive | Decrease | t(29) = −0.83 | 0.41 | −0.31 | ||
| Increase | Decrease | t(29) = −0.62 | 0.54 | −0.23 | ||
Note: SZ = Schizophrenia patients; CN = Healthy controls
Figure 6. Grand Average SP Waveform Amplitude for the Decrease Condition.
Note: The Figure demonstrates that the decrease instruction impaired conflict monitoring in CN (as indicated by higher SP amplitude for congruent than incongruent); however, this effect was absent in SZ, presumably because they could not decrease negative emotion effectively using reappraisal. SP = Sustained Potential; SP amplitude is evaluated between 750 – 900 ms post-Stroop onset. The scalp maps demonstrates that the SP was observed in the expected electrodes in both groups.
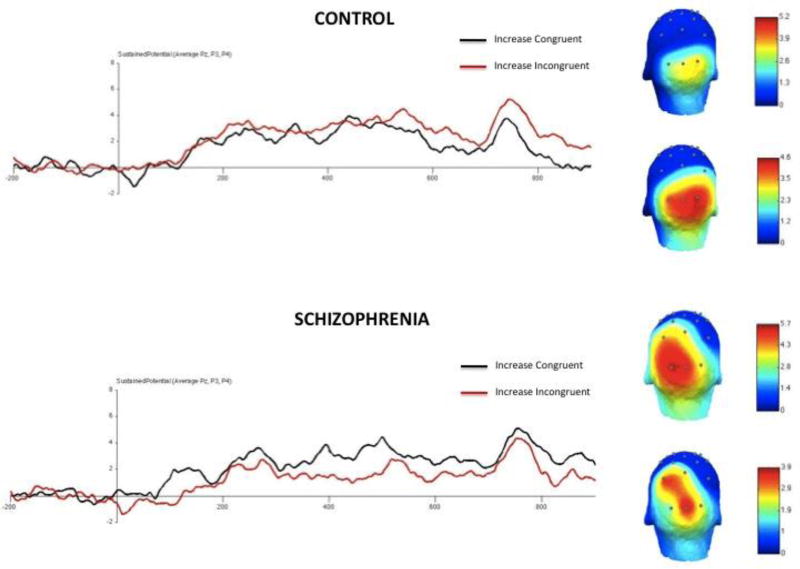
Figure 7. Grand Average SP Waveform Amplitude for the Increase Condition.
Note. The Figure demonstrates that the increase instruction enhanced conflict monitoring in CN (as indicated by higher SP amplitude for incongruent than congruent); however, this effect was not observed in SZ patients, who showed a trend toward increase instructions impairing conflict monitoring. SP = Sustained Potential; SP amplitude is evaluated between 750 – 900 ms post-Stroop onset. The scalp maps demonstrates that the SP was observed in the expected electrodes in both groups.
Correlations Between Task Performance and Clinical Outcomes
Bivariate Pearson’s correlations were conducted within SZ to examine the relationship between clinical variables and key task variables (see Table 6). Trait negative affect was significantly correlated with LPP amplitude in all three of the negative stimulus conditions, but not with LPP amplitude in the neutral condition. Disorganized Symptoms were significantly associated with Stroop reaction time interference score in the Decrease condition. However, none of these correlations survive bonferroni correction. There were no significant correlations in CN.
Table 6.
Correlations between Emotion Regulation and Clinical Variables in Schizophrenia
| PANAS PA |
PANAS NA |
BNSS Tot |
BPRS Pos |
BPRS Neg |
BPRS Dis |
BPRS Tot |
LOF Tot |
|
|---|---|---|---|---|---|---|---|---|
| Decrease RT Interference | −0.09 | 0.06 | −.27 | .22 | −.27 | 0.51** | 0.17 | 0.10 |
| Increase RT Interference | 0.01 | −0.08 | 0.02 | 0.07 | −0.07 | 0.20 | 0.13 | 0.24 |
| Unpleasant Passive RT Interference | 0.09 | −0.09 | 0.07 | 0.02 | 0.01 | 0.28 | 0.11 | 0.05 |
| Neutral RT Interference | 0.02 | −0.12 | −0.06 | 0.14 | −0.23 | 0.23 | 0.02 | 0.15 |
| SP Neutral Congruent | 0.19 | −0.08 | −0.08 | 0.09 | 0.22 | 0.11 | 0.13 | −0.08 |
| SP Neutral Incongruent | 0.17 | 0.09 | −0.17 | 0.07 | 0.27 | 0.10 | 0.28 | −0.04 |
| SP Unpleasant Passive Congruent | 0.13 | 0.06 | 0.04 | 0.05 | 0.12 | 0.02 | 0.03 | −0.02 |
| SP Unpleasant Passive Incongruent | 0.07 | 0.01 | −0.23 | 0.06 | 0.05 | 0.29 | 0.18 | −0.10 |
| SP Increase Congruent | 0.12 | 0.01 | 0.07 | −0.15 | 0.15 | −0.22 | −0.07 | 0.06 |
| SP Increase Incongruent | 0.17 | −0.05 | −0.04 | 0.07 | 0.09 | 0.04 | 0.05 | −0.27 |
| SP Decrease Congruent | 0.29 | −0.18 | −0.05 | −0.01 | 0.12 | 0.04 | 0.03 | −0.16 |
| SP Decrease Incongruent | 0.16 | −0.01 | −0.35 | 0.02 | −0.05 | 0.26 | 0.05 | 0.07 |
| LPP Neutral | 0.11 | −0.08 | −0.15 | 0.11 | −0.16 | −0.12 | −0.15 | 0.01 |
| LPP Unpleasant Passive | −0.31 | 0.45* | 0.05 | 0.34 | −0.12 | 0.05 | 0.15 | −0.32 |
| LPP Increase | −0.25 | 0.43* | −0.06 | 0.29 | −0.21 | −0.18 | −0.10 | −0.04 |
| LPP Decrease | −0.16 | 0.45* | −0.16 | 0.22 | −0.18 | −0.08 | 0.03 | −0.07 |
Note. RT = Reaction Time; Interference = Incongruent – Congruent; LPP = Late Positive Potential; PANAS = Positive and Negative Affect Scale; BNSS = Brief Negative Symptom Scale; BPRS = Brief Psychiatric Rating Scale; LOF = Level of Function Scale
Discussion
Several important findings emerged in the current study. As expected, CN were able to decrease the neurophysiological response to unpleasant stimuli via reappraisal. However, this was not true of SZ patients. Although SZ patients demonstrated significantly higher LPP amplitude in the unpleasant passive viewing than neutral condition, consistent with intact emotional reactivity, they failed to reduce LPP amplitude using decrease instructions. These findings are consistent with results of two prior ERP studies which found that a reappraisal manipulation failed to decrease LPP amplitude to unpleasant stimuli (Horan et al., 2013; Strauss et al., 2013), as well as prior fMRI studies demonstrating aberrant frontal activation during reappraisal (Morris et al., 2012; van der Meer et al., 2014). These ERP and fMRI findings provide converging evidence that SZ patients have difficulty implementing cognitive change strategies to decrease negative emotion.
The current findings on decreasing negative emotion also extend results of prior reappraisal studies in two important ways. First, the two prior imaging studies that found ineffective implementation of reappraisal both used a cognitively demanding reinterpretation tactic (Morris et al., 2012; van der Meer et al., 2014). In the current study, we observed that patients also failed to effectively implement a less cognitively demanding distancing tactic (Ochsner et al., 2011). Given the magnitude of cognitive deficits in SZ, one would expect patients to be impaired on a highly demanding task; however, it is informative that patients are also impaired on those that are less demanding, as it suggests that even the simpler forms of reappraisal may be too complex for patients to successfully implement. Since both reinterpretation and distancing tactics are used in psychosocial interventions for emotion regulation, these findings suggest that strategies other than reappraisal, particularly those that are less cognitively demanding, may be worth exploring in treatment. Second, in the two prior ERP cognitive change studies (Horan et al., 2013; Strauss et al., 2013), the emotion regulation manipulation administered to participants was more implicit in nature. An audio clip was presented that described an upcoming unpleasant image as being more neutral, thereby providing the participant with a reappraisal of the stimulus that they did not have to self-generate. In the current study, participants were required to self-generate their own reappraisal of the unpleasant stimulus, which is a more explicit process. Growing evidence that SZ patients are impaired on both implicit and explicit tasks is important because it suggests that difficulties with reappraisal are not simply due to being unable to follow instructions or self-generate appropriate reappraisals. The emotion regulation impairment appears to result from abnormalities operating during the process of implementation itself.
There are several potential explanations for how and where the breakdown in implementation could occur. Reappraisal is a cognitively complex process that involves several neural substrates (Ochsner et a., 2011). For example, participants must first allocate attention toward features of a stimulus that are being appraised and gate that information into working memory- processes that rely on dorsolateral and posterior portions of the prefrontal cortex and inferior parietal regions. Next, they must inhibit their initial prepotent appraisal of the stimulus and select a more goal-appropriate reappraisal, which relies on activation of the ventrolateral prefrontal cortex. In instances where the stimulus being reappraised depicts other humans engaged in social interactions, participants will also perform mental state attributions and activate the dorsomedial prefrontal cortex. Finally, conflict monitoring processes must also be engaged to determine whether the reappraisal attempted was effective at changing emotional response as intended, relying on dorsal regions of the anterior cingulate. Any of these components of the reappraisal process could contribute to implementation failures given that SZ patients display both cognitive impairments and structural/functional abnormalities in all of these areas. Future neuroimaging studies are needed that manipulate individual components of the reappraisal process (e.g., attending to stimuli and gating into working memory, inhibiting prepotent appraisals, mental state attribution, conflict monitoring) to determine which cognitive and neural processes are most directly contributing to ineffective implementation and which are intact. This will be an important first step in determining how existing emotion regulation interventions that use psychosocial or computerized training programs should be modified to target the precise mechanisms involved with ineffective implementation.
There was also evidence for areas of spared emotion regulation performance in SZ. This was the first study to show that both CN and SZ are able to increase the neurophysiological response to unpleasant stimuli using reappraisal. The dissociation between intact ability to increase negative affect and impaired ability to decrease negative affect via reappraisal may be informative. Distinct areas of the prefrontal cortex support increase and decrease goals (see Ochsner et al., 2011 for a review). Specifically, although increasing and decreasing negative emotion recruit left prefrontal cortex (PFC) regions, decreasing additionally recruits right prefrontal cortex regions significantly more than increasing (Eippert et al., 2007; Ochsner et al., 2004; Urry et al., 2006). Decreasing negative emotion may therefore require more anatomical substrates and presumably additional cognitive resources not used to increase negative emotion. It is possible that SZ patients do not recruit these additional cognitive processes and neural substrates while implementing decrease instructions. Future neuroimaging studies should directly explore this possibility.
Most importantly, results of the current study indicated that increase and decrease instructions modulated the SP in opposite directions in CN; however, SZ patients did not display this pattern. The literature on the SP component in healthy individuals indicates that good conflict monitoring is indicated by significantly higher amplitude for incongruent than congruent trials (i.e., a positive difference score) (West., 2003). Our CN group displayed higher SP amplitude for incongruent than congruent trials in the increase condition, indicating that increase instructions enhanced cognitive control. This finding is consistent with the beneficial effects of increasing negative emotion observed in a study of healthy undergraduates by Moser, Most, and Simons (2010). In contrast, SZ patients did not show the same benefit of the increase instruction, despite being able to increase negative emotion via reappraisal successfully. Rather, SZ displayed a significantly lower SP difference score than CN, suggesting that increase instructions impaired conflict monitoring. A different pattern of results occurred in the decrease condition. CN displayed higher SP amplitude for congruent than incongruent trials, suggesting that the decrease instruction impaired conflict monitoring. These CN findings are contrary to Moser et al. (2010) who found no effect of decrease instruction on the SP. Inconsistent results across studies may reflect differences in CN sample demographics (i.e., community CN’s in the current study were older, more likely to be male, less educated, and had lower IQ than the undergraduate sample of Moser et al.). In contrast, SZ patients displayed no difference in SP amplitude for congruent and incongruent conditions following the decrease instruction, presumably because they could not decrease negative emotion effectively. Thus, while findings suggest that using reappraisal to increase negative emotion enhances cognitive control and decreasing negative emotion impairs it in CN, these effects were not evident in SZ patients. Effectively increasing negative emotion impairs cognitive control and ineffectively decreasing negative emotion has no effect in SZ. The differential effects of increase and decrease instructions on SP interference scores may reflect unique neural circuits involved with these instructions (Eippert et al., 2007; Ochsner et al., 2004; Ochsner et al., 2012; Urry et al., 2006). Future fMRI studies should test this hypothesis.
Collectively, these SP findings have important implications. They suggest that emotion regulation ability has a direct, causal effect on cognitive control operations performed immediately after emotion regulation strategies are attempted. Heightened negative emotion is common in SZ (Horan et al., 2008) and patients may attempt to combat these experiences by frequently implementing emotion regulation strategies. These efforts may not only be ineffective, but the current results suggest that they may also be cognitively “costly”, depleting already limited cognitive resources and detrimentally affecting information processing. Although future research is needed to replicate and extend the current findings, our results suggest that emotion regulation abnormalities may therefore play an important and under recognized role in generalized neurocognitive impairment in SZ. Emotion regulation interventions may be an intriguing supplemental treatment for cognitive control impairments in SZ that could be added on to cognitive rehabilitation programs. Since cognitive control and emotion regulation processes rely on similar neural circuitry, improvements in one domain may have a synergistic effect on the other.
Several limitations should be considered when interpreting these findings. First, although sample size was adequate to detect robust ERP effects, there was insufficient power to detect behavioral effects. ERP measures may be more sensitive than behavioral measures in detecting conflict monitoring effects on the Stroop task. Second, although ERPs provide excellent temporal resolution, their spatial resolution is poor. We proposed several neuroanatomical explanations for the differences between increase and decrease conditions; however, future neuroimaging studies are needed to directly explore theses tentative hypotheses. Third, we did not explore the effects of high vs low arousal unpleasant stimuli on cognitive control. Previous findings suggest that reappraisal is most effective with lower arousal unpleasant stimuli while other strategies (e.g., distraction) are more effective for higher arousal stimuli (Sheppes et al., 2011; Sheppes et al., 2014). On average, unpleasant stimuli in this task were moderate to high arousal. It is unknown whether SZ patients can effectively increase and decrease neurophysiological response to lower arousal unpleasant stimuli using reappraisal, and whether differing levels of arousal have different effects on cognitive control. Third, although the present design examined the effects of reappraisal on cognitive control, the relationship between these two variables is likely bi-directional. Cognitive control and reappraisal rely on overlapping neural circuits in the prefrontal cortex, and it is likely that reappraisal problems could stem from impairments in basic cognitive control processes. Emotion regulation abnormalities may simply reflect one of the many ways that control deficits manifest in SZ. Finally, the effects of antipsychotic medications should be considered. The current sample contained a somewhat higher proportion of unmedicated patients than what is usual for studies of stabilized, chronically ill patients. This was due to sampling strategy (community and TV advertisements) that resulted in enrollment of several (n = 10) patients who were not currently prescribed antipsychotics, stably outside of the treatment system, and functioning reasonably well. Although these individuals had better vocational outcome and lower negative symptom severity than patients prescribed antipsychotics, there were no significant group × medication status interactions for primary task variables. Chlorpromazine equivalent dosage was also not significantly correlated with primary task variables. Thus, although the unmedicated patients had better functioning medicated patients, antipsychotics did not appear to effect task performance; however, it should be noted that although these analyses are fairly standard methods for determining antipsychotic effects, they are relatively crude estimates of the role of antipsychotics. Conclusions about antipsychotic effects should therefore be made with these caveats in mind.
In summary, despite these limitations, several key findings emerged. First, SZ can effectively increase, but not decrease the neurophysiological response to unpleasant stimuli using a cognitive distancing reappraisal tactic. Second, increase instructions enhanced cognitive control in CN participants, but impaired cognitive control in SZ patients. In contrast, decrease instructions impaired cognitive control in CN participants, but had no effect on cognitive control in SZ. These findings may suggest that abnormalities in emotion regulation play an under-recognized role in cognitive control impairments in SZ.
Acknowledgments
Research supported by NIMH Grant K23-MH092530 to Dr. Strauss
Footnotes
The percentage of trials removed after artifact rejection was: SZ: Unpleasant passive = 12%, Neutral = 13%, Decrease = 12%, Increase = 14%; CN: Unpleasant passive = 8%, Neutral = 10%, Decrease = 9%, Increase = 10%; SZ: Congruent = 12%; Incongruent = 13%; CN: Congruent = 9%; Incongruent = 10%.
Analyses were also conducted on the N450. All main effects and interactions were nonsignificant.
We reconducted all analyses on the primary dependent variables comparing medicated and unmedicated patient groups. Medication Group × Condition interactions were nonsignificant for all variables of interest: RT interference scores, accuracy interference scores, LPP scores, and SP interference scores. There were no significant correlations between chlorpromazine equivalent dosage and the aforementioned task variables. Thus, antipsychotics do not appear to have a major influence on task variables reported in the main analyses. As might be expected, the medicated patients had significantly higher total (F [1, 29] = 8.92, p < 0.01), expressive (F [1, 29] = 7.74, p = 0.01), and motivation/pleasure (F [1, 29] = 5.8, p < 0.03) negative symptoms than unmedicated patients; however, there were no significant differences between medication groups in positive (F [1, 29] = 1.1, p = 0.30), disorganized (F [1, 29] = 0.01, p = 0.91), or total (F [1, 29] = 1.82, p = 0.18) BPRS symptoms. There were also no significant differences in total (F [1, 29] = 3.04, p = 0.09) or social (F [1, 29] = 0.38, p = 0.54) functional outcome between medicated and unmedicated groups; however, unmedicated patients were more likely to have better work outcomes than medicated patients (F [1, 29] = 4.54, p < 0.05). Thus, medicated patients primarily differed from unmedicated patients in vocational functioning and negative symptom severity, but antipsychotics had no apparent effect on task performance.
References
- Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical psychology review. 2010;30(2):217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Badcock JC, Paulik G, Maybery MT. The role of emotion regulation in auditory hallucinations. Psychiatry Research. 2011;185(3):303–308. doi: 10.1016/j.psychres.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Archives of general psychiatry. 2001;58(3):280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Barch DM, Sheffield JM. Cognitive impairments in psychotic disorders: common mechanisms and measurement. World Psychiatry. 2014;13(3):224–232. doi: 10.1002/wps.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EA, Gross JJ, Barnard K. Testing the effects of suppression and reappraisal on emotional concordance using a multivariate multilevel model. Biological psychology. 2014;98:6–18. doi: 10.1016/j.biopsycho.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, Malaspina D. The stress cascade and schizophrenia: etiology and onset. Schizophrenia bulletin. 2003;29(4):671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human brain mapping. 2007;28(5):409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Foti D, Hajcak G. Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience. 2008;20(6):977–988. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998;2(3):271–299. [Google Scholar]
- Gross JJ. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39(3):281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The extended process model of emotion regulation: elaborations, applications, and future directions. Psychological Inquiry. 2015;26(1):130–137. [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Hawk AB, Carpenter WT, Strauss JS. Diagnostic criteria and five-year outcome in schizophrenia: A report from the International Pilot Study of Schizophrenia. Archives of general psychiatry. 1975;32(3):343–347. doi: 10.1001/archpsyc.1975.01760210077005. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Simons RF. Attending to affect: Appraisal strategies modulate the electrocortical response to arousing pictures. Emotion. 2006;6(3):517–522. doi: 10.1037/1528-3542.6.3.517. [DOI] [PubMed] [Google Scholar]
- Henry JD, Rendell PG, Green MJ, McDonald S, O'Donnell M. Emotion regulation in schizophrenia: Affective, social, and clinical correlates of suppression and reappraisal. Journal of Abnormal Psychology. 2008;117(2):473–478. doi: 10.1037/0021-843X.117.2.473. [DOI] [PubMed] [Google Scholar]
- Horan WP, Blanchard JJ, Clark LA, Green MF. Affective traits in schizophrenia and schizotypy. Schizophrenia Bulletin. 2008;34(5):856–874. doi: 10.1093/schbul/sbn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Hajcak G, Wynn JK, Green MF. Impaired emotion regulation in schizophrenia: evidence from event-related potentials. Psychological Medicine. 2013;43(11):2377–2391. doi: 10.1017/S0033291713000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D, Vakhrusheva J, Jobson-Ahmed L, Tarrier N, Malaspina D, Gross JJ. Emotion awareness and regulation in individuals with schizophrenia: Implications for social functioning. Psychiatry Research. 2012;200(2–3):193–201. doi: 10.1016/j.psychres.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Strauss GP, Linh N, Fischer BA, Daniel DG, Cienfuegos A, Marder SR. The brief negative symptom scale: Psychometric properties. Schizophrenia Bulletin. 2011;37(2):300–305. doi: 10.1093/schbul/sbq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- Lansbergen MM, van Hell E, Kenemens JL. Impulsivity and conflict in the Stroop task: An ERP study. Journal of Psychophysiology. 2007;21(1):33–50. [Google Scholar]
- Livingstone K, Harper S, Gillanders D. An Exploration of Emotion Regulation in Psychosis. Clinical Psychology & Psychotherapy. 2009;16(5):418–430. doi: 10.1002/cpp.635. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Frontiers in human neuroscience. 2014;8(4):1–14. doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markela-Lerenc J, Schmidt-Kraepelin C, Roesch-Ely D, Mundt C, Weisbrod M, Kaiser S. Stroop interference effect in schizophrenic patients: an electrophysiological approach. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2009;71(3):248–257. doi: 10.1016/j.ijpsycho.2008.10.005. [DOI] [PubMed] [Google Scholar]
- McNeely HE, West R, Christensen BK, Alain C. Neurophysiological evidence for disturbances of conflict processing in patients with schizophrenia. Journal of Abnormal Psychology. 2003;112(4):679–688. doi: 10.1037/0021-843X.112.4.679. [DOI] [PubMed] [Google Scholar]
- Morris RW, Sparks A, Mitchell PB, Weickert CS, Green MJ. Lack of cortico-limbic coupling in bipolar disorder and schizophrenia during emotion regulation. Translational Psychiatry. 2012;2 doi: 10.1038/tp.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Most SB, Simons RF. Increasing negative emotions by reappraisal enhances subsequent cognitive control: A combined behavioral and electrophysiological study. Cognitive Affective & Behavioral Neuroscience. 2010;10(2):195–207. doi: 10.3758/CABN.10.2.195. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JEGDR. The brief psychiatric rating scale. Psychological Reports. 1962;10(3):799–812. [Google Scholar]
- Pantelis C, Barnes TRE, Nelson HE, Tanner S, Weatherley L, Owen AM, Robbins TW. Frontal-striatal cognitive deficits in patients with chronic schizophrenia. Brain. 1997;120:1823–1843. doi: 10.1093/brain/120.10.1823. [DOI] [PubMed] [Google Scholar]
- Perry Y, Henry JD, Grisham JR. The habitual use of emotion regulation strategies in schizophrenia. British Journal of Clinical Psychology. 2011;50:217–222. doi: 10.1111/j.2044-8260.2010.02001.x. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality: SIDP-IV. American Psychiatric Pub; 1997. [Google Scholar]
- Raine A, Lencz T, Reynolds GP, Harrison G, Sheard C, Medley I, Cooper JE. An evaluation of structural and functional prefrontal deficits in schizophrenia: MRI and neuropsychological measures. Psychiatry Research-Neuroimaging. 1992;45(2):123–137. doi: 10.1016/0925-4927(92)90006-p. [DOI] [PubMed] [Google Scholar]
- Rowland JE, Hamilton MK, Vella NC, Lino BJ, Mitchell PB, Green MJ. Adaptive associations between social cognition and emotion regulation are absent in schizophrenia and bipolar disorder. Frontiers in psychology. 2013;3:607. doi: 10.3389/fpsyg.2012.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppes G, Scheibe S, Suri G, Gross JJ. Emotion regulation choice. Psychological Science. 2011;22:1391–1396. doi: 10.1177/0956797611418350. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Suri G, Gross JJ. Emotion regulation and psychopathology. Annual review of clinical psychology. 2015;11:379–405. doi: 10.1146/annurev-clinpsy-032814-112739. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Ruiz I, Dickinson EK, Frost KH. A review of the role of negative emotion abnormalities in anhedonic symptoms of schizophrenia. In: Sangha S, Foti D, editors. Emotion and Motivated Behaviors: Integrating Animal and Human Neurobiology Research. New York: Oxford University Press; in press. [Google Scholar]
- Strauss GP, Kappenman ES, Culbreth AJ, Catalano LT, Lee BG, Gold JM. Emotion regulation abnormalities in schizophrenia: cognitive change strategies fail to decrease the neural response to unpleasant stimuli. Schizophrenia Bulletin. 2013;39(4):872–883. doi: 10.1093/schbul/sbs186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Kappenman ES, Culbreth AJ, Catalano LT, Ossenfort KL, Lee BG, Gold JM. Emotion regulation abnormalities in schizophrenia: Directed attention strategies fail to decrease the neurophysiological response to unpleasant stimuli. Journal of abnormal psychology. 2015;124(2):288. doi: 10.1037/abn0000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchselvam R, Blechert J, Sheppes G, Rydstrom A, Gross JJ. The temporal dynamics of emotion regulation: An EEG study of distraction and reappraisal. Biological Psychology. 2011;87(1):84–92. doi: 10.1016/j.biopsycho.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Urry HL, Van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience. 2006;26(16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L, Swart M, van der Velde J, Pijnenborg G, Wiersma D, Bruggeman R, Aleman A. Neural correlates of emotion regulation in patients with schizophrenia and non-affected siblings. PloS One. 2014;9(6):e99667–e99667. doi: 10.1371/journal.pone.0099667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L, van't Wout M, Aleman A. Emotion regulation strategies in patients with schizophrenia. Psychiatry Research. 2009;170(2–3):108–113. doi: 10.1016/j.psychres.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu. Rev. Clin. Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- West R. Neural correlates of cognitive control and conflict detection in the Stroop and digit-location tasks. Neuropsychologia. 2003;41(8):1122–1135. doi: 10.1016/s0028-3932(02)00297-x. [DOI] [PubMed] [Google Scholar]