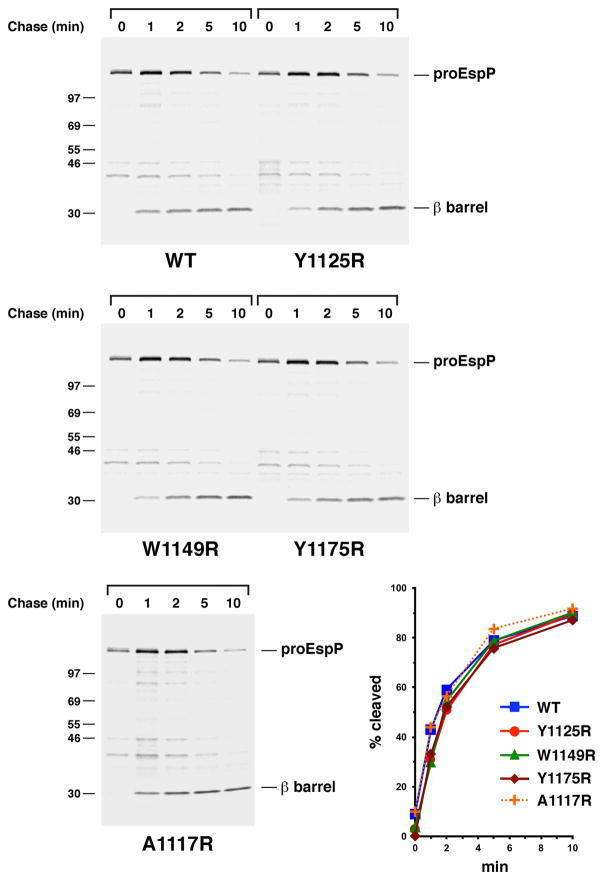
Fig. 6.
The introduction of a lipid-facing arginine residue near the extracellular or periplasmic side of the EspP β barrel does not significantly affect assembly. AD202 transformed with pRLS5 (Ptrc-espP) or a pRLS5 derivative encoding the indicated EspP mutant were subjected to pulse-chase labeling after the addition of IPTG. Immunoprecipitations were then conducted using an antiserum generated against an EspP C-terminal peptide. The percentage of the passenger domain that was released from the β domain by proteolytic cleavage at each time point is shown.