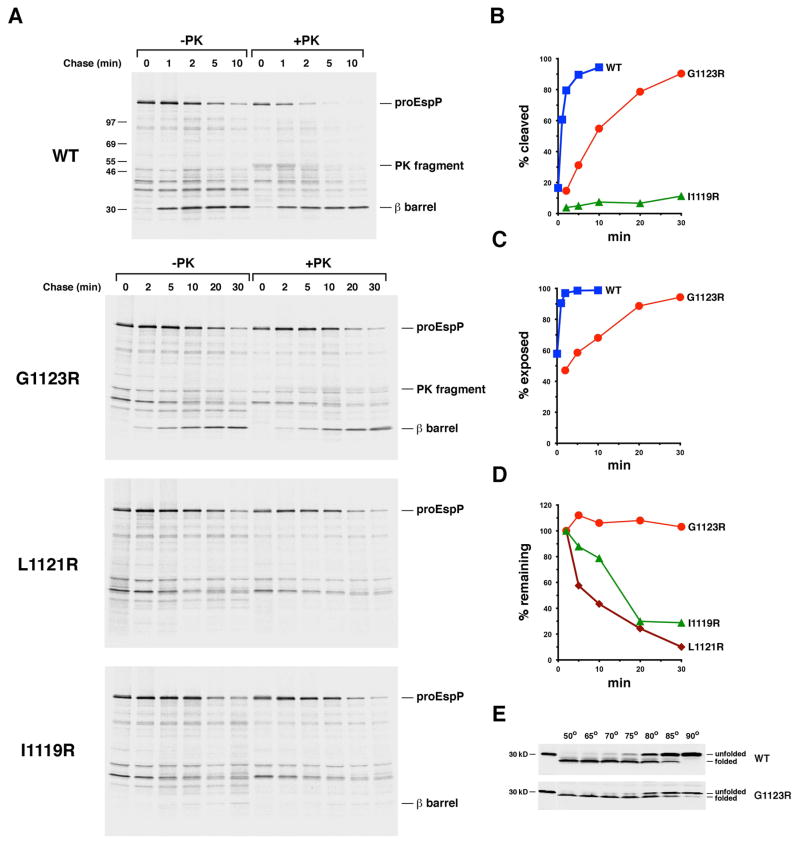
Fig. 7.
The introduction of a lipid facing arginine residue near the middle of the EspP β barrel delays or blocks membrane integration. A. AD202 transformed with pRLS5 (Ptrc-espP) or a pRLS5 derivative encoding the indicated EspP mutant were subjected to pulse-chase labeling after the addition of IPTG. Half of the cells were treated with PK, and immunoprecipitations were conducted using an antiserum generated against an EspP C-terminal peptide. The percentage of the passenger domain that was released from the β domain by proteolytic cleavage or surface exposed at each time point is plotted in (B) and (C). The percentage of the radiolabeled protein that remained at each time point is shown in (D). E. Cell membranes isolated from AD202 that produced wild-type EspP or the G1123R mutant were heated at the indicated temperature in SDS-PAGE sample buffer and the free β barrel domain was detected by Western blot.