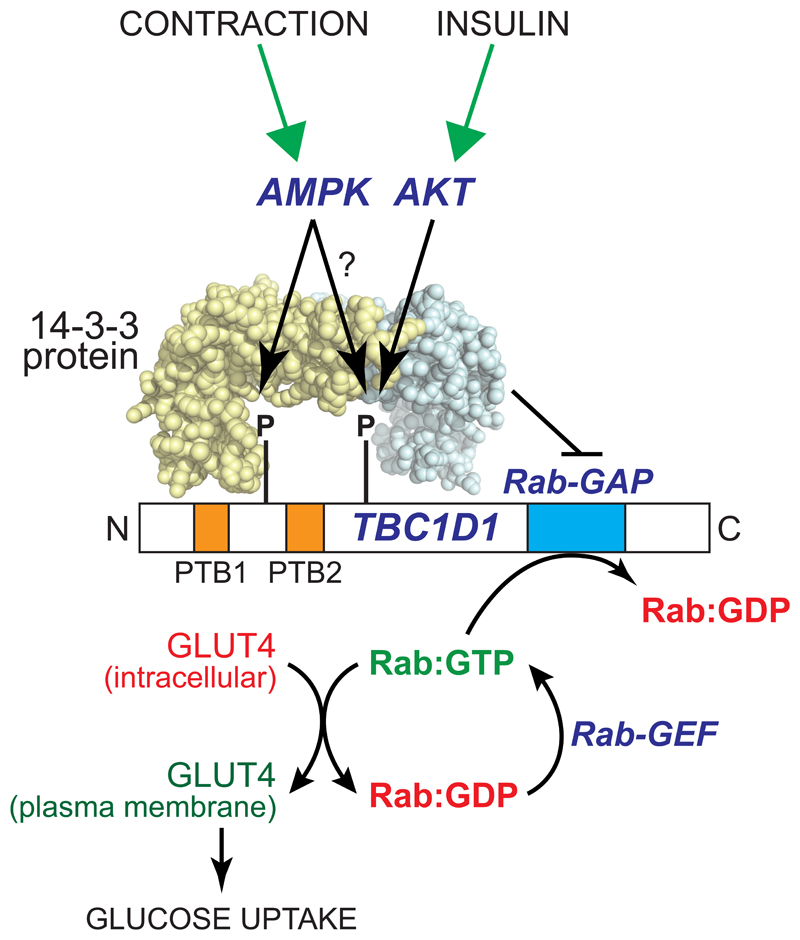
Figure 3. Model for acute activation of glucose transport in muscle by AMPK.
In the unphosphorylated form, the protein TBC1D1 retains GLUT4 at intracellular sites because its Rab-GAP domain promotes the inactive GDP-bound state of members of the Rab family of small G proteins. AMPK phosphorylates TBC1D1 at Ser237 near the PTB1 domain, while Akt (and perhaps also AMPK?) phosphorylates Thr596 near PTB2. This dual phosphorylation promotes the binding of 14:3:3 proteins, abundant dimeric proteins containing two symmetrical pockets that bind to phosphorylated peptides, which is proposed to inhibit the Rab-GAP activity of TBC1D1. The functions of the two phosphotyrosine-binding (PTB) domains of TBC1D1 remain unclear.