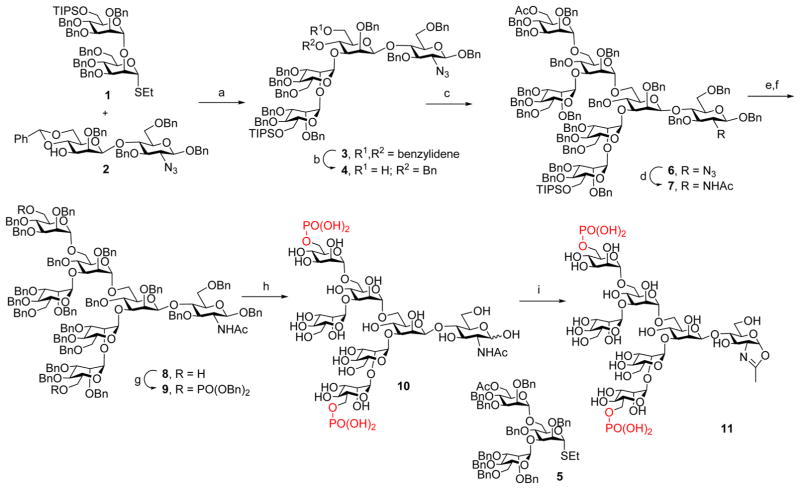
Scheme 1. Synthesis of the Bis-M6P Heptasaccharide Oxazoline (11)a.
aReagents and conditions: (a) NIS, TfOH, MS4A, Et2O, −40 °C to rt, 76%. (b) Et3SiH, PhBCl2, 4 Å molecular sieves (MS4A), CH2Cl2, −78 °C, 85%. (c) 5, NIS, AgOTf, MS4A, 0 °C to rt, 97%. (d) AcSH, pyridine, CHCl3, rt, 86%. (e) NaOMe, MeOH, THF, rt. (f) TBAF, THF, rt, 75% in two steps. (g) (BnO)2PiPr2, tetrazole, MS4A, CH2Cl2 then mCPBA, 87%. (h) Pd–C, H2, THF, MeOH then Pd(OH)2–C, H2, MeOH, H2O, quant. (i) DMC, Et3N, H2O, rt, 90%.