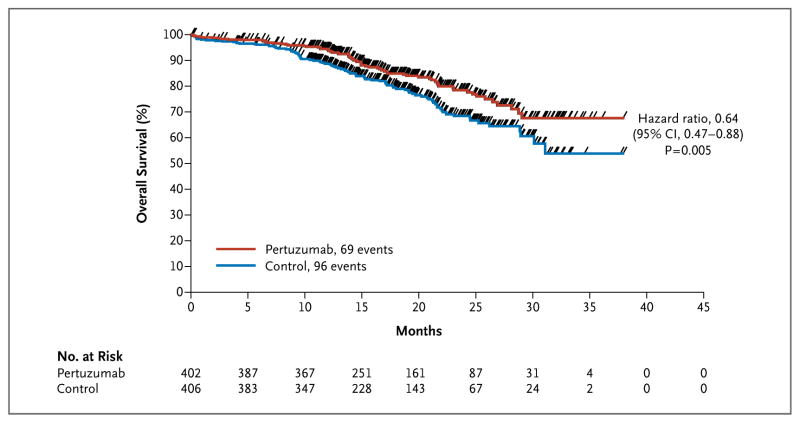
Figure 2. Overall Survival.
Kaplan–Meier estimates of overall survival in patients in the intention-to-treat population are shown. The tick marks indicate the times at which events were recorded. The interim overall survival analysis was performed after 165 events (43% of the prespecified total number for the final analysis) had occurred: 96 events in the control group (placebo plus trastuzumab plus docetaxel) and 69 events in the pertuzumab group (pertuzumab plus trastuzumab plus docetaxel). The interim analysis of overall survival did not cross the O’Brien–Fleming stopping boundary threshold; therefore, the interim result is not statistically significant and is deemed exploratory.