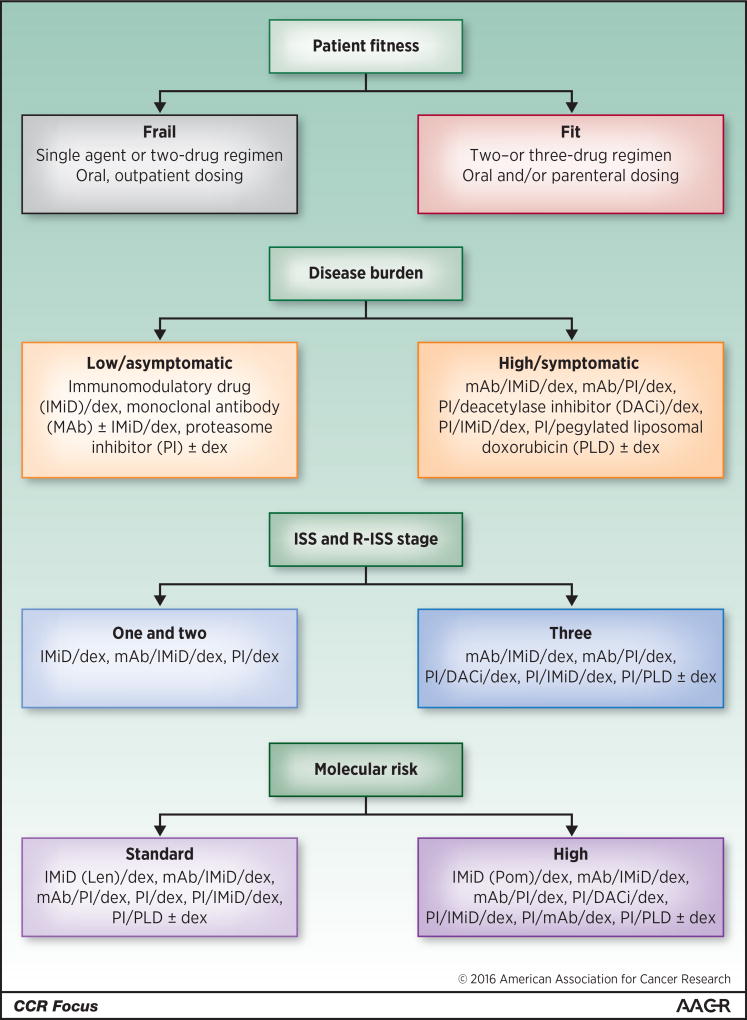
Figure 1. Factors to be wighed in deciding on therapy for patients with multiple myeloma.
Patient fitness, disease burden, International Staging System (ISS) and Revised-ISS stage, and molecular risk should be considered in making treatment decisions for myeloma patients in either the newly-diagnosed, relapsed, or refractory settings. However, randomized controlled trials (RCTs) trials addressing the use of these factors have largely not been performed. These suggestions are therefore grounded in the clinical experience of the authors, and are not as evidence-based as we would ideally like. Also, practitioners will need to keep in mind the settings for which these drugs have or have not been approved. In particular, many of the options, including some of the immunomodulatory drugs (IMiDs), pegylated liposomal doxorubicin (PLD), proteasome inhibitors (PIs), monoclonal antibodies (MAbs), and the deacetylase inhibitor (DACi) are not approved for front-line therapy. Finally, the options are presented alphabetically, and their order should not be construed as a suggestion of their appropriate sequencing, since such studies have not been performed. Additional abbreviation: dex, dexamethasone.