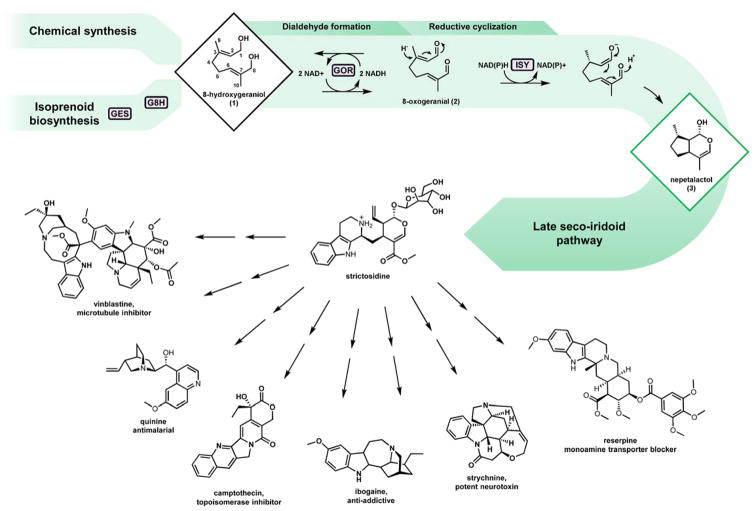
Fig. 1. Monoterpene indole alkaloid biosynthesis.
The pathway intermediate 8-hydroxygeraniol 1 may be produced via chemical synthesis or de novo via isoprenoid biosynthesis. De novo production proceeds via the monoterpene precursor geranyl pyrophosphate, which may be hydrolyzed and ω-hydroxylated by GES and G8H respectively. The resulting diol 1 is converted by GOR to the dialdehyde 8-oxogeranial 2, which undergoes reductive cyclization by ISY. The iridoid 3 scaffold is then subject to a number of oxidations, methylation, glucosylation, and condensation with tryptamine to generate the universal monoterpene indole alkaloid (MIA) precursor strictosidine. Several MIAs with important biological activities are shown.