Figure 1. Defects in Aire cooperate with defects in peripheral tolerance to promote autoimmunity.
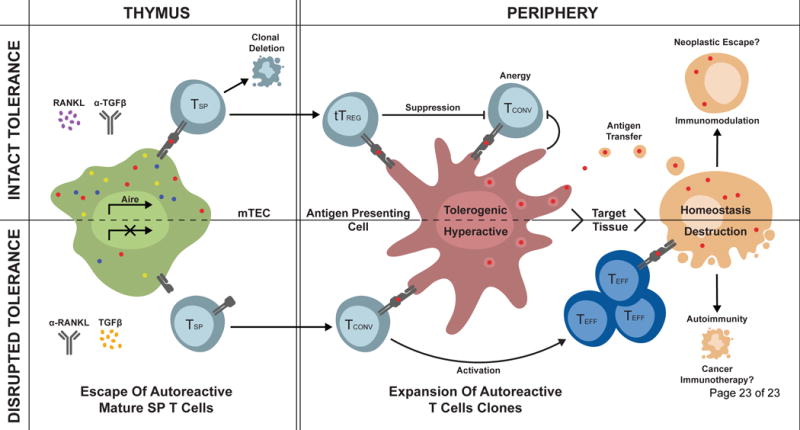
Aire induces expression of TSAs in thymic mTECs, which are presented to developing single positive (SP) T cells. Self-reactive T cells that bind peptide-MHC complexes with high affinity are either deleted by negative selection or become thymically derived natural tTregs. Mutations in the Aire gene leading to reduced or absent AIRE function result in decreased TSA presentation and survival and thymic escape of autoreactive T-cell clones as well as a loss of tTreg induction. Autoreactive T-cells can then encounter cognate self-antigen presented by APCs in secondary lymphoid organs or peripheral tissues. When peripheral tolerance checkpoints are intact, autoreactive T cells are suppressed by inhibitory signals from Tregs as well as resting APCs. Failure of peripheral tolerance leads to their activation and autoimmune attack. RANKL and TGFβ are two factors known to influence mTEC function, and thereby, AIRE and TSAs expression. Therapies directly targeting these molecules or their downstream pathways may provide therapeutic opportunities for immune modulation in the context of hyperactivity or release of tolerance in the context of neoplastic transformation and cancer immunotherapy.
