FIGURE 1.
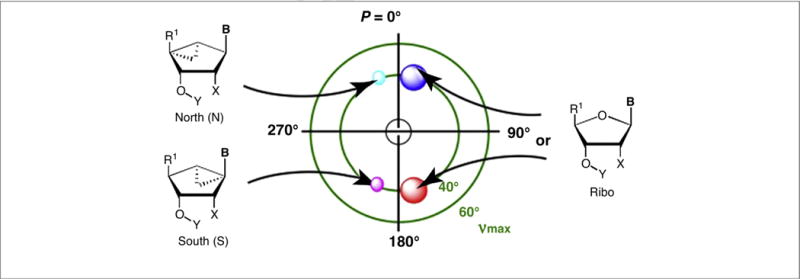
Relationship between ribose ring structure and favored conformations as depicted on the pseudorotational cycle, made by a mathematical formula to describe all twists of the ribose ring [5]. P = pseudorotational angle; ν = out of plane angle. On right side: red circle = region of North (N) conformation in nature; with cyan and link circles representing the conformations of the methanocarba rings (left side). Typically: B = nucleobase; Y = H; X = OH. R1 can be oxymethylene or carbonyl moieties.
