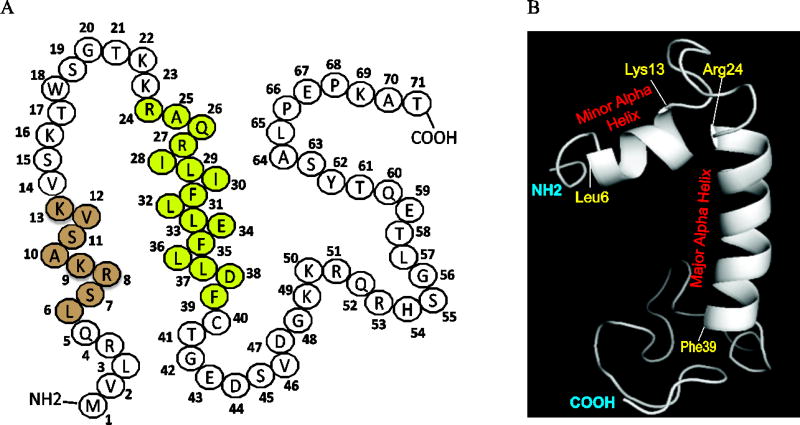
Fig. 1. Structural features of JCV Agno.
(A) Primary amino acid sequence composition of JCV agnoprotein, highlighting location of the minor (leu6-Lys13, light brown colored) and major (Lys24-Phe39, light green colored) alpha helical regions. Agno is a highly basic protein in nature containing many Lys and Arg residues on its N- and C-terminus regions. The major alpha helix is however, mainly composed of hydrophobic residues. (B) A recently revealed NMR structure of the JCV full-length Agno which highlights the location of the minor (Leu6-Lys13) and major alpha helix (Lys24-Phe39) regions and the intrinsically unstructured (Met1-Gln5, Val14-Lys23 and Cyt40-Thr71) domains.