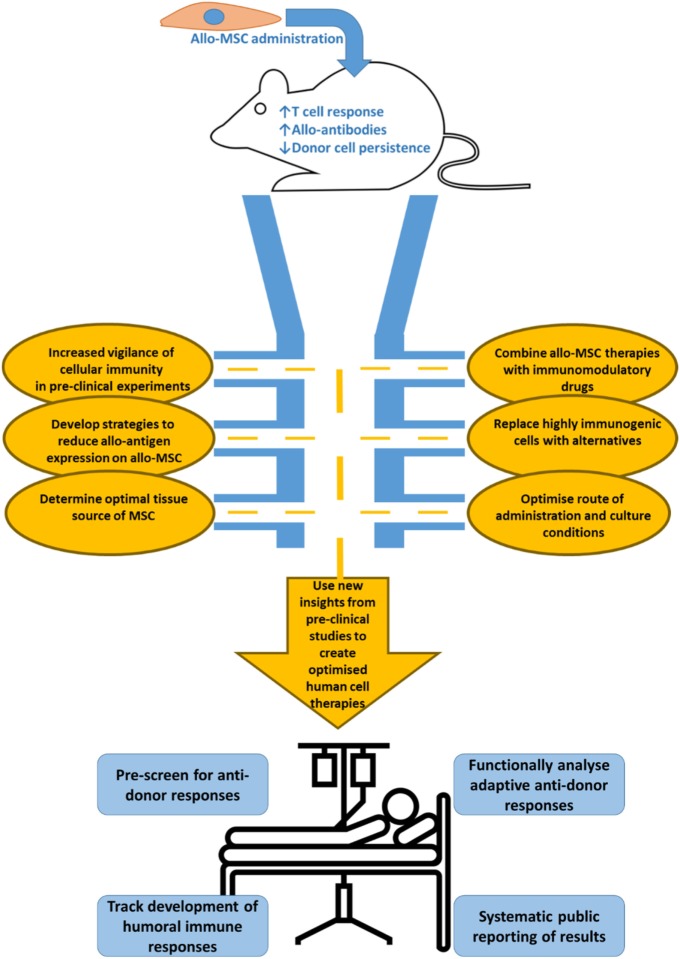
Figure 1.
Immunological considerations for the development of next generation allo-MSC therapy. Allogeneic administration of MSC in a variety of pre-clinical models has been shown to induce allo-immune responses. These potentially harmful responses include generation of memory T cells, production of functional allo-antibodies and allo-specific clearance of administered cells. More evidence on the specific effects of allo-MSC administration on a model specific basis is required however and the field would benefit from systematic reporting of relevant allo-immune reactions elicited by MSC in pre-clinical models. The development of the next generation of MSC therapies will require focused and multi-disciplinary pre-clinical experiments to (1) fully characterize and report cell mediated immunity, (2) if necessary, to lower these immune responses in the recipients by working on synergy of allo-MSC with immunomodulatory drugs, (3) by developing strategies to reduce allo-antigen expression on administered cells by using genetic manipulation or small molecules, and (4) if appropriate, replacing highly immunogenic MSC cell therapies with alternatives. In addition to developing bespoke indication specific strategies like those mentioned above, the field as a whole should look to optimize the source, culture conditions and routes of administration of MSC in order to minimize immunogenic reactions to the cells while maintaining efficacy. In the coming years, more detailed pre-clinical data will allow lessons to be learned to create optimized allo-MSC therapies for clinical use. However, we should continue to carefully monitor the allo-immune response once allo-MSC are administered to the patient. This surveillance should include pre-screening patients for pre-existing anti-donor immune responses, monitoring anti-donor cellular and humoral immune responses and or equal importance systematic reporting of results in easily available formats.