Figure 1.
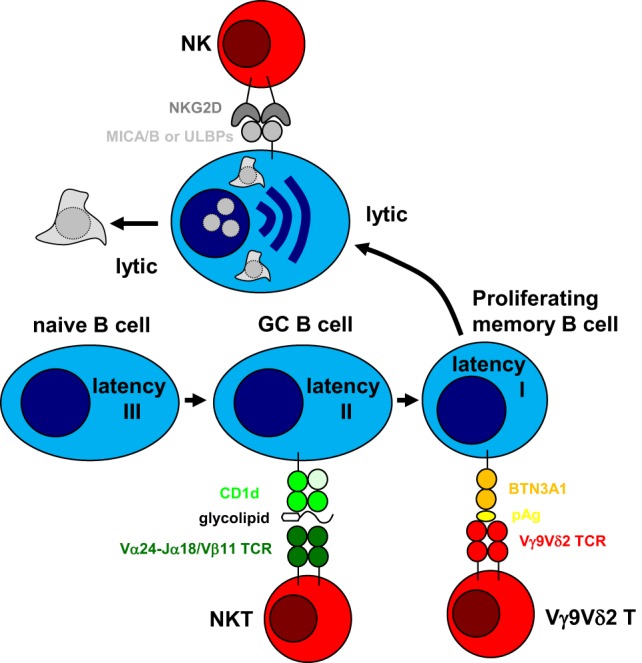
Innate lymphocytes target different stages of Epstein–Barr virus (EBV) infection. EBV was suggested to drive B cell differentiation by expressing all eight latent EBV proteins (latency III) in tonsillar naïve B cells and rescuing germinal center (GC) B cells with the expression of three latent EBV proteins (latency II) toward memory B cells. In homeostatically proliferating memory B cells, only one latent EBV protein is expressed for viral genome maintenance (latency I). From this infected memory B cell pool, EBV can reactivate into virus producing lytic replication, most likely after B cell receptor engagement. Natural killer (NK) cells have been shown to preferentially recognize lytic EBV replication and NKG2D has been suggested as an activating receptor involved in this recognition after upregulation of its MICA/B and ULBP ligands. In a subgroup of infected individuals, Vγ9Vδ2 T cells can be stimulated by EBV latency I Burkitt’s lymphoma cell lines and recognize these by mevalonate metabolite recognition in a butyrophilin (BTN) 3A1-dependent fashion. Finally, natural killer T (NKT) cells have been suggested to recognize EBV latency II in Hodgkin’s lymphoma cell lines, presumably by recognizing glycolipid presentation on CD1d. Thus, cytotoxic innate lymphocytes can target different stages of EBV infection.
