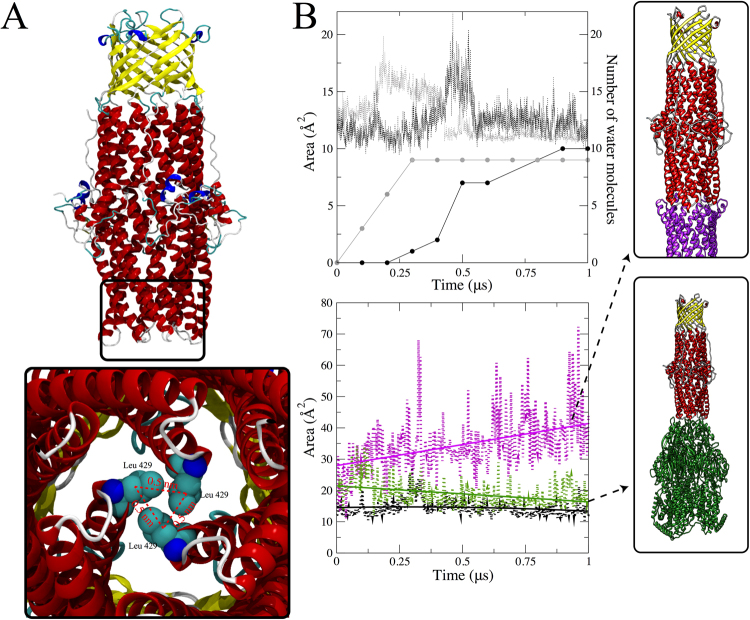
Figure 4.
MexA-dependent opening of the OprM periplasmic aperture. (A) Structure of OprM highlighting the constraints in the periplasmic tips. Three helices are packed together and enclose three hydrophobic leucine residues (Leu429; one per OprM protomer) with an α-carbon distance value of 0.5 nm between each pair of leucines. These three leucine residues are used as reference points for computing a triangular-shaped area (dotted red lines) for monitoring the aperture area. (B) Top panel: Spontaneous partial opening that leads to water translocation through the closed state of OprM. Two independent simulations (black and grey dotted lines) reveal stochastic partial opening with an increase of ~5 Å2 that allows the permeation of 10 water molecules. This process occurs within 1 µs of the simulation (black and grey dotted lines). Bottom panel: OprM aperture opening is activated by the close interactions between the outer membrane and periplasmic proteins (purple). The aperture opening was computed as a function of the enclosed triangular shaped area as given in (A). As a reference, the average area of closed OprM (black) is also provided. Aperture opening of OprM is not fully triggered by the interaction with the inner component MexB (green) and remains only in a partially opened state.