Abstract
Gliomas are CNS neoplasms that infiltrate the surrounding brain parenchyma, complicating their treatment. Tools that increase extent of resection while preventing neurological deficit are essential to improve prognosis of patients diagnosed with gliomas. Tools such as intraoperative MRI, ultrasound and fluorescence-guided microsurgery have been used in the surgical resection of CNS gliomas with the goal of maximizing extent of resection to improve patient outcomes. In addition, emerging experimental techniques, for example, optical coherence tomography and Raman spectroscopy are promising techniques which could 1 day add to the increasing armamentarium used in the surgical resection of CNS gliomas. Here, we present the potential advantages and limitations of these imaging techniques for the purposes of identifying gliomas in the operating room.
KEYWORDS : fluorescence-guided resection, intraoperative MRI, intraoperative ultrasound, optical coherence tomography, Raman spectroscopy
Gliomas are CNS neoplasms that infiltrate the surrounding brain parenchyma. This is a characteristic that complicates treatment of intrinsic brain tumors and is shared by low-grade (LGG, WHO grade II) and high-grade gliomas (HGGs, WHO grades III and IV). An increasing amount of evidence suggests that extent of resection is an important determinant of the outcome of patients diagnosed with LGGs and HGGs [1–6]. The determination of the tumor border using preoperative imaging techniques or intraoperative observations has proven to be challenging due to the heterogeneity of these tumor and because of the presence of cells infiltrating far away from contrast-enhancing regions seen in preoperative MRI studies; which are also invisible during microsurgical resection of gliomas. Hence, the development of techniques and tools to enhance the extent of surgical resection in patients with LGG and HGG is extremely important. Tools such as intraoperative MRI (iMRI), ultrasound (US), fluorescence-guided microsurgery, optical coherence tomography (OCT) and Raman spectroscopy (RS) are now part of the increasing armamentarium used in the surgical resection of gliomas with the goal of maximizing extent of resection to improve patient outcomes. Since the advent of these technologies, neurosurgeons have sought to implement them as part of the limited number of tools available to treat these difficult and devastating neoplasms. They have also been the subject of study of a multitude of investigators attempting to determine which is the best adjunct during surgical resection leading to survival and quality of life benefit.
Intraoperative US
The use of intraoperative US (IOUS) was introduced into the neurosurgical operating room in the 1950s initially as a lesioning device. When real-time ultrasonographic imaging capabilities were developed, the US became an important tool in the neurosurgical operating room [7–9]. Because of the great evolution in image quality and US equipment, IOUS has been used to treat a wide variety of intracranial tumors (Figure 1) [10–13]. IOUS is more available than other intraoperative imaging modalities because it is an inexpensive tool existent in places with limited resources. The utility of IOUS has been studied in glioma surgery. Woydt et al. studied whether IOUS was able to determine the presence of tumor tissue after surgical resection. This was correlated with histopathological analysis of tissue samples obtained after the surgeon had obtained what was determined to be a gross-total resection [14]. In a study including 38 patients with HGG and 9 with LGG, the authors concluded that if IOUS found a rim of more than 3 mm of hyperechogenicity, this was likely to be residual tumor, whereas if the rim of hyperechogenicity was smaller than 3 mm, the finding was not specific for the presence of residual tumor [14]. Other authors have found that the specificity and sensitivity were low before and after resection of the tumor, which can be caused by US artifact [15,16]. In a study of 156 patients that underwent surgery for HGG, Solheim et al. found that the effectiveness of 3D IOUS during surgical resection of gliomas is significantly affected by the quality of US images obtained. Additionally, the value of this imaging modality is decreased by the presence of hemorrhage and edema in the tumor and in the surrounding tissues [12]. However, IOUS provides real-time imaging of the neoplasm in an attempt to bypass the shift of tissues that occurs throughout surgery [13]. Other groups have evaluated the utility of a navigable 3D IOUS system in the resection of HGG. In a series of 90 patients with intracranial tumors (of which 51 were HGG and 17 were LGG), Moiyadi et al. found that the use of the IOUS improved resection in 59% of the patients operated and in 21%, the US showed residual tumor that was not resected due to the vicinity with eloquent tissue. The rate of gross-total resection was found to be 88% when this was evaluated in the group of tumors that were considered resectable. They also discuss that IOUS was helpful in identifying nearby vascular structures using Doppler angiography [17].
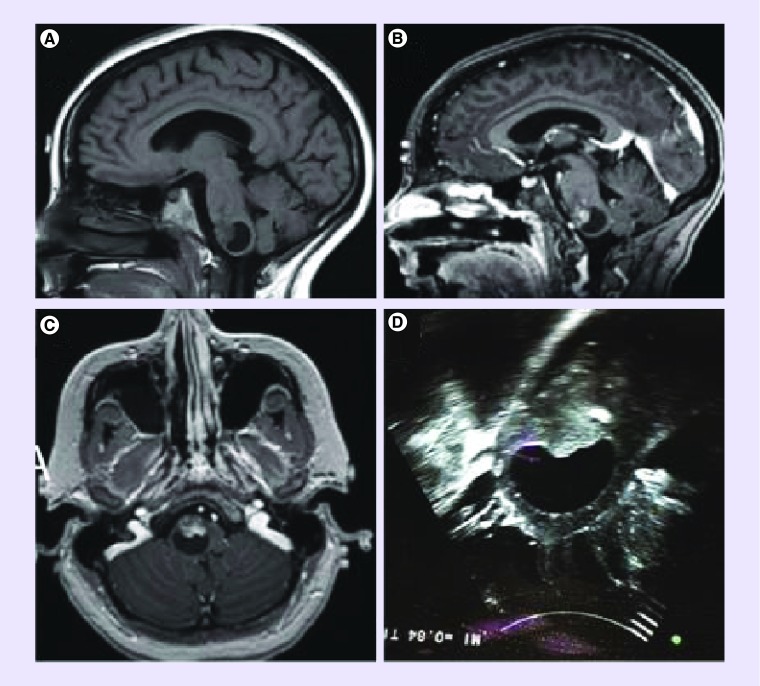
Figure 1. . MRI scan and intraoperative ultrasound of a patient with a brainstem pilocytic astrocytoma.
(A) Sagittal T1 without gadolinium; (B) Sagittal T1 with gadolinium; (C) axial T1 with gadolinium; (D) intraoperative ultrasound.
In LGG surgery, IOUS has been useful in the identification of tumor and residual tissue. Le Roux, presented a series of 33 patients with LGG who underwent surgical resection aided with IOUS where it was possible to identify residual tissue and the authors concluded that this tool may enhance extent of resection of LGG [18]. Similarly, another group presented a series of 35 patients with parenchymal lesions, 11 of which were LGG and 22 were HGG [19]. The conclusions of this study were similar to the ones obtained by Le Roux, showing that IOUS was helpful in identifying intraparenchymal lesions and identifying its borders to enhance resection [19]. In a study of 32 patients with intra-axial tumors (15 of which were gliomas) where extent of resection has been evaluated in postoperative imaging studies, it was reported that a rim of 5 mm of hyperechoic tissue was predictive of residual tumor in 100%, but there was a negative predictive value of 83% when correlated with postoperative MRI [20]. Several other studies have addressed the rate of gross total resection (GTR) in glioma patients. The reported range of GTR ranges from 57 to 95% in the studies reviewed [12,17,20–25]. In a comprehensive analysis of a large number of studies, a meta-analysis by Mahboob et al. demonstrated that IOUS-guided resection of gliomas is a valuable tool by increasing extent of resection [26]. Ultimately, some studies indicate that the active use of IOUS results in increase in survival time and progression-free survival (PFS) in glioblastoma (GBM) patients [27,28]. In a study evaluating the quality of life of GBM patients, IOUS proved to improve quality of life by reducing the incidence of postoperative deficits [29].
A different modality employing US contrast agents, contrast-enhanced US (CEUS) has also been used in the resection of gliomas. US contrast agents are materials that have different echogenicity and provide better quality images of the tissue and definition of tumor borders. This IOUS modality can also be coupled to a navigable US machine. In a study of ten patients that underwent surgery for resection of GBM, Prada et al. showed that the use of CEUS is feasible and has the potential to locate residual tumor [30]. In a study of 50 patients with a variety of brain tumors including gliomas, metastases and meningiomas, other studies have also shown feasibility and usefulness of CEUS with good delineation of residual tumors particularly useful for GBM. Although CEUS is able to define tumor borders in gliomas, there are significant technical variables that may present as an obstacle for implementation of CEUS due to the extensive experience required for its use [31,32].
IOUS is a widely available and inexpensive tool that is helpful in the resection of HGG and LGG, among other intraparenchymal brain lesions. Further studies and advances in technology will enhance the usefulness of IOUS in resection of CNS tumors. The results of significant studies are summarized in Table 1. However, it is extremely important to mention that IOUS has limitations, which are mainly user dependent. User-dependent limitations occur due to the knowledge, skill and practice that the neurosurgeon has using this intraoperative imaging modality. Therefore, the usefulness of this imaging modality is highly dependent on the comfort and experience of the operator. Other limitations include but are not limited to image quality, difficulties with edema and hemorrhage within tumors and absence of functional data.
Table 1. . Summary of significant studies evaluating different intraoperative imaging modalities for glioma surgery.
| Study (year) | Type of study | Intraoperative imaging modality | Patient population | Conclusion | Ref. |
|---|---|---|---|---|---|
| Intraoperative ultrasound | |||||
| Moiyadi et al. (2015) | Prospective | Navigable US | 88 gliomas, 32 GBM | Use of navigable US improved progression-free survival and overall survival | [27] |
| Saether et al. (2012) | Restrospective | IOUS | 192 patients, GBM | Survival improved after implementation of IOUS | [28] |
| Jakola et al. (2011) | Retrospective | IOUS | 63 high-grade glioma patients and 25 low-grade glioma patients | Use of IOUS preserves quality of life | [29] |
| Intraoperative MRI | |||||
| Senft et al. (2011) | Prospective, randomized controlled trial | iMRI | 29 patients in iMRI group and 29 patients in conventional surgery group | More patients in the iMRI group had gross-total resection | [36] |
| Kubben et al. (2014) | Prospective randomized controlled trial (interim analysis) | Ultralow field iMRI vs neuronavigation | 14 patients | No difference in extent of resection and survival | [38] |
| Coburger et al. (2015) | Retrospective | High-field iMRI | 199 patients | Progression-free and overall survival in GBM patients undergoing surgery with iMRI have improved since it was initially introduced | [37] |
| Coburger et al. (2015) | Prospective | iMRI + 5-ALA | 33 patients iMRI + 5-ALA, 144 controls iMRI alone | Significant increase in extent of resection in the iMRI + 5-ALA group compared with iMRI alone | [41] |
| Fluorescence-guided microsurgery | |||||
| Yamada et al. (2015) | Prospective | 5-ALA + iMRI | 97 patients, WHO III and IV gliomas | Improved identification of tumor tissue beyond contrast enhancement | [53] |
| Díez Valle et al. (2011) | Prospective | 5-ALA | 36 patients, GBM | Enhanced resection of contrast-enhancing tissue | [50] |
| Roder et al. (2014) | Retrospective | 5-ALA vs iMRI | 117 patients, GBM | iMRI is superior to 5-ALA guided surgery | [52] |
| Schucht et al. (2014) | Prospective | 5-ALA + motor mapping | 67 patients, GBM | Gross-total resection of enhancing tumor tissue in 73% of patients in spite of close proximity to corticospinal tract | [63] |
| Koc et al. (2008) | Prospective | Fluorescein | 47 patients with fluorescein-guided surgery and 33 patients without FGM | Fluorescein use increased gross-total resection from 55% (no FGM) to 83% (FGM) | [58] |
| Neira et al. (2016) | Prospective | Fluorescein | 32 patients, GBM | Fluorescein is a good marker of tumor tissue in contrast-enhancing and noncontrast enhancing regions | [59] |
5-ALA: 5-aminolevulinic acid; FGM: Fluorescence-guided microsurgery; GBM: Glioblastoma; iMRI: Intraoperative MRI; IOUS: Intraoperative ultrasound; US: Ultrasound.
Intraoperative MRI
iMRI was initially developed in 1997. In their study, Black et al. used iMRI to treat a wide range of intracranial lesions (Figure 2) [33]. Since then it has been used as part of the neurosurgical armamentarium to determine presence of residual tumor and also to circumvent the presence of shift after the dura has been opened and tumor has been resected when neuronavigation is being used. Since its introduction to the neurosurgical operating room, MRI machines have evolved together with image quality [34]. This intraoperative imaging modality has improved extent of resection and, in turn, patient outcomes by providing information regarding residual tumor in the operative bed obtained during the surgery in the treatment of HGG as well as LGG [35]. Additionally, neuronavigation can be updated during surgery with the information obtained from iMRI, bypassing the shift after cerebrospinal fluid drainage, dural opening and/or tumor removal.
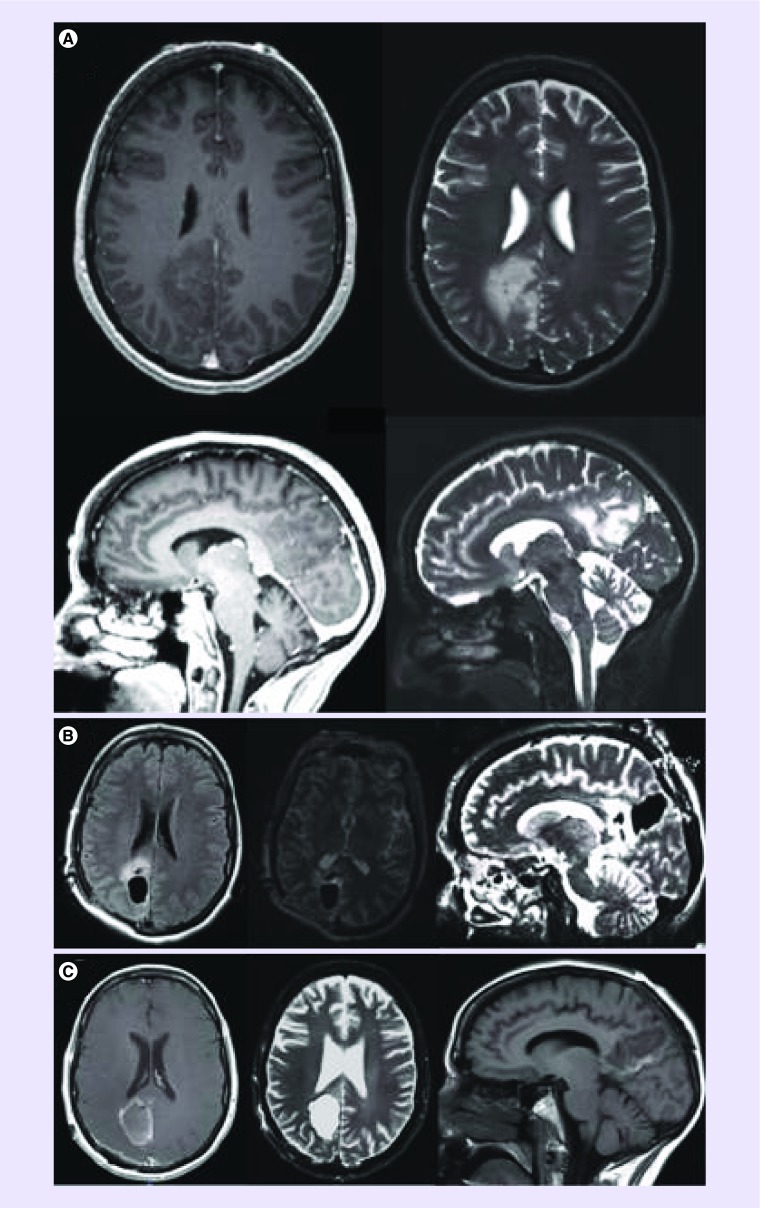
Figure 2. . Pre and postoperative images after resection of a low grade glioma using intraoperative MRI.
(A) Preoperative MRI demonstrating a right medial occipitoparietal masswith minimal contrast enhancement in the left upper and lower panels in T1 post-contrast image. The mass demonstrates T2 hyper attenuation in the right upper and lower pannels. (B) Intraoperative MRI images during resection of the right medial occipitoparietal mass (left panel-axial T1 sequence, middle panel-axial T2 sequence, right-sagittal T2 sequence). (C) Postoperative MRI demonstrating result of resection of right medial occipitoparietal mass.
In the only randomized controlled trial evaluating the benefit or iMRI in glioma surgery, Senft et al. provided the only level I evidence to date to support iMRI use in glioma surgery. In a series of 58 patients randomized to iMRI-aided resection or conventional neuronavigation, show that a higher percentage of the patients included in the iMRI arm had GTR than the group in which navigation was performed. Additionally, the rate of new neurological deficit was similar between both groups and extent of resection correlated with better survival, but being in the iMRI group did not result in improved survival [36]. In a recent series of 170 patients treated with high-field iMRI, Coburger et al. assessed rate of GTR, overall survival, PFS and incidence of new neurological deficits after surgery [37]. These parameters were compared with previous reports and the authors concluded that GTR and overall survival were better, whereas complication rate was lower than previously reported [37]. In a randomized controlled trial designed to compare the role of iMRI versus standard neuronavigation, it was found that there was no benefit in extent of resection or in survival for patients who had iMRI-guided surgery [38]. However, this study only included 14 patients and also utilized ultralow field MRI [38]. In a systematic review of multiple studies addressing the use of iMRI for surgical GBM treatment, Kubben et al. concluded that the use of iMRI during GBM surgery improves extent of resection (EOR) as well as PFS. However, some of the drawbacks of this analysis of existing studies include the heterogeneity of populations and criteria to determine EOR, the different characteristics of MRI equipment used in the studies, among others. In a meta-analysis performed by Eljamel et al., patients with HGG undergoing iMRI had a GTR rate of 70% [39]. The authors analyzed the utility of IOUS, IMRI and fluorescence-guided surgery. All these modalities showed comparable rates of GTR in meta-analyses performed for each imaging technique, with slightly higher GTR rate for the fluorescein-guided resections [39].
Studies on LGGs are not as prevalent. In the case of LGG surgery, iMRI has an accuracy to detect residual tumor of 83%. iMRI performed better at detecting residual tumor tissue than IOUS. These findings were confirmed histologically [40].
Studies that combine iMRI with 5-aminolevulinic acid (5-ALA) fluorescence-guided surgery have shown an increased EOR as compared with iMRI alone, but there was no benefit in survival. [41,42]. Some of the limitations of iMRI include the high cost of the equipment as well as the need for special infrastructure [43]. Additionally, MRI does require that the surgery be stopped while image acquisition occurs, extending the surgical time. Moreover, the identification of tumor margins is defined by their imaging characteristics resulting in radiological GTR, while there is residual neoplastic tissue that cannot be identified by this imaging modality. The correlation between the presence of contrast enhancement in iMRI and the presence of histopathological diagnosis of neoplastic tissue is not perfect. Although contrast enhancement correlates with tumor presence, the absence of contrast enhancement does not correlate with tumor absence [44]. The results of significant studies are summarized in Table 1. It is conceivable that by combining multiple intraoperative techniques to identify tumor tissue the extent of resection may be improved further. This is ultimately important if the increase in EOR is accompanied with a survival benefit and the incidence of postoperative neurological deficits decreases.
Fluorescence-guided resection of gliomas
FGM to treat intrinsic brain tumors is based on the ability to label tumoral tissue with fluorescent agents such as protoporphyrin IX (PpIX) and fluorescein. PpIX-induced fluorescence has been used to guide surgical resection of gliomas after administration of its precursor 5-ALA. 5-ALA accumulates in tumor cells and hijacks the heme synthesis pathway to produce PpIX. PpIX accumulates in cancer cells through the activity and transported into cancer and normal cells by oligopeptide transporter 1 or 2 (PEPT1 or PEPT2) [45] and can be excited with blue–violet light generating the emission of red light [46]. 5-ALA has been approved for patient use in Europe, however, it has not been approved by the US FDA, except for centers with research protocols in place.
A significant amount of attention has been directed toward FGM, mainly toward 5-ALA technique, due to the simplicity of the technique. It has been tested in a randomized controlled trial that included 322 patients [47], where 65% of patients in the 5-ALA group received GTR compared with 36% of patients in the control group. Additionally, 41% of patients in the 5-ALA group had 6-month PFS compared with 21% in the control group. There were no statistically significant differences in survival between 5-ALA and control groups. Since then, multiple studies show the benefit of ALA-FGM in the treatment of HGG in increasing EOR, and combination with other tools such as intraoperative mapping techniques allows for safer resections [48–53]. In a systematic review by Zhao et al., ALA-FGM is better than conventional neuronavigation in the treatment of HGG by improving EOR and improving survival [54].
In 1999, Kuroiwa et al. developed a surgical microscope equipped to detect fluorescein fluorescence and they used it to help in HGG resection [55]. Fluorescein was administered systemically and it accumulated in regions with faulty blood–brain barrier. After resection, they observed that areas of high fluorescence correlated with areas with high tumor cell density histologically. Availability of microscopes equipped with fluorescein filters has allowed the implementation of this technique [55–57]. Also, the low cost of use of fluorescein makes it a more cost-effective tool when compared with 5-ALA [39]. Since then, in 2008 Koc et al. executed a study evaluating the influence of fluorescein-FGM in the treatment of HGG [58]. They found that GTR was greatly improved with the use of fluorescein. Recently, Neira et al. demonstrated in a study of 32 patients that fluorescence was present in all patients after fluorescein administration. They also were able to achieve GTR in 93% of patients with an average resected volume of 99.7% [59]. Currently, fluorescein is being evaluated as an intraoperative brain tumor marker in a clinical trial, however it is not currently approved for brain tumor surgery by the FDA (clinical trial NCT02691923).
In a meta-analysis comparing different intraoperative imaging techniques in the resection of HGG, the patients included in the ALA-FGM group, there was a 61.9% of patients with no residual contrast enhancing tissue in postoperative imaging, whereas 84.4% of patients included in the fluorescein-FGM did not have residual contrast-enhancing tissue [39]. The rate of GTR in this meta-analysis in the IOUS and iMRI were 73.6 and 70%, respectively. The authors also determined that the cost of ALA-FGM was of approximately $1400, the cost of fluorescein-FGM was $300 and IOUS and iMRI were $330 and $1800, respectively. Additionally the specificity and sensitivity of ALA-FGM and fluorescein-FGM were found to be much better than those for IOUS and iMRI. Improvement in these techniques may allow for increased sensitivity in the detection of tumor tissue. The EOR achieved with ALA-FGM can be greatly improved by the use of PpIX-spectroscopy given that this technique is three-times more sensitive than the commercial microscopes equipped with fluorescence filters [60].
Although the use of FGM techniques improves EOR, the ultimate proof is the improvement in survival. In a meta-analysis, ALA-FGM and fluorescein-FGM showed an estimated survival improvement of 4 months after ALA-FGM [49] and approximately 2.1 weeks after fluorescein-FGM [58]. In this study, GTR did have a significant effect on improvement of survival and fluorescein-FGM patients had 83% GTR versus 55% of patients who did not have fluorescein-FGM.
Limitations of fluorescein-FGM include skin photosensitivity, hypotension and the limitation of its use in color blind surgeons [61,62]. Photosensitivity can be prevented through light protection after 5-ALA administration and hypotension can be avoided by identifying patients with cardiac disease. Fluorescein-FGM can be hampered by hypersensitivity [60]. Other potential pitfalls of this technique include the inadvertent extension of the resection to eloquent regions. This can be avoided by combining FGM with intraoperative mapping during glioma resection [63]. The results of significant studies are summarized in Table 1. Ultimately, by combining multiple technologies, with the combination tailored to the individual patient's characteristics, we may see improvement in outcomes and results in glioma resection. For example, in a tumor nearby eloquent structures, ALA or fluorescein-FGM in conjunction with intraoperative monitoring may be the most appropriate option.
Experimental technique: RS
RS is another relatively new experimental imaging technology which has been introduced into the operating room. Although it has not yet received FDA approval, it has shown promising pilot results in detecting tumor cells in vivo in mice and in humans. RS is a technique used to identify vibrational, rotational and other low-frequency modes. As a result, it can characterize tissues by observing the molecular fingerprint for tumor versus nontumor. The intraoperative feasibility of this technology was first tested by a Canadian group in 2015 with 17 patients [64], and the results are promising for spot detection of brain cancer during surgical resection. Nevertheless, limitations for RS include a small field of view at 0.00000025 mm2 area to 1 mm3 volume/spot. Furthermore, it has a slow scanning rate at 1 s/spot, and therefore cannot be used to scan the entire resection cavity within the surgical timeframe [64,65] (Table 2).
Table 2. . Summary of studies evaluating experimental intraoperative imaging modalities for glioma surgery.
| Study (year) | Type of study | Imaging modality | Study population | Conclusion | Ref. |
|---|---|---|---|---|---|
| Raman imaging | |||||
| Ji et al. (2013) |
Ex vivo mice In vivo mice Ex vivo human |
Stimulated Raman scattering microscopy | Frozen sections in 12 mice In vivo imaging in 16 mice Ex vivo human surgical specimen in 1 patient |
Strong correlation when compared with H&E microscopy for detection of glioma infiltration (κ = 0.98) | [65] |
| Jermyn et al. (2015) | In vivo human | Raman spectroscopy | 17 patients with grade II–IV glioma | Raman-based probe differentiates normal brain cancer with 93% sensitivity and 91% specificity | [64] |
| Optical coherence tomography | |||||
| Bizheva et al. (2005) | Ex vivo human (formalin fixed) | OCT | Formalin-fixed tissues from 3 patients (meningioma, paraganglioma) | First OCT study to study histology features in human brain cancer | [72] |
| Bohringer et al. (2006) |
Ex vivo mouse Ex vivo human (formalin fixed) |
OCT | Formalin-fixed tissues from 1 mouse model and 1 human biopsy specimen | First OCT study to extract quantitative attenuation data in ex vivo mouse and human brain specimens | [68] |
| Böhringer et al. (2009) | In vivo human | OCT | 9 patients with high-grade glioma (grade III, IV) | First OCT study to extract attenuation data from in vivo human brain cancer | [71] |
| Kut et al. (2015) |
In vivo mice Ex vivo human (fresh surgical specimens) |
OCT; optical property mapping | 5 in vivo mice 32 glioma patients (grade II-IV) |
First systematic study with established diagnostic threshold for cancer vs noncancer. Blinded validation study showed high sensitivity/specificity (92/100% for high-grade, 100/80% for low-grade glioma) | [76] |
H&E: Hematoxylin and eosin; OCT: Optical coherence tomography.
Experimental technique: OCT
OCT is a label-free, real-time imaging technique which can be used to obtain volumetric images of biological tissues at a resolution equivalent to a low-powered microscope (e.g., around two- to fourfold magnification). OCT can be envisioned as an optical analog of US imaging, since both techniques acquire cross-sectional images of the tissues by collecting ‘reflected’ light or sound waves. Unlike US, however, OCT uses a near-infrared light source (instead of sound waves), and does not use any matching medium, for example, gels as used in US imaging. In addition, OCT is capable of noncontact imaging and generally acquires images at several centimeters above the tissue surface, which minimizes the risks of infection. Since its introduction about two to three decades ago, OCT has evolved to become a powerful medical imaging technique with the unique ability to visualize the cross-sectional structures of human tissues noninvasively, at high speed, and with micron-level resolution [66]. OCT was first used in ophthalmological applications to detect retina pathologies. Since then, it has become successful in multiple clinical specialties [66–70], with FDA approval for ophthalmic, gastrointestinal and intravascular applications.
Recent groups have made important contributions to the study of human brain cancer using OCT [67,69–74]. For example, Boppart et al. performed the first OCT study in imaging ex vivo human brain cancer in 2008, where the research group has imaged a melanoma tumor mass which had metastasized to the cerebral cortex of the patient [67]. In 2005, Bizheva et al. obtained the cross-sectional OCT images of formalin-fixed human brain cancer tissues such as meningioma and ganglioglioma [72]. In 2013, Assayag et al. performed the first en face OCT studies in human brain cancer specimens [74]. Finally, Bohringer et al. reported the results of several pilot studies which characterized the optical attenuation characteristics of human primary brain cancers ex vivo and in vivo [68,71,75] (Table 2).
A study in Science Translational Medicine (published in June 2015) built upon previous studies and investigated the potential of OCT for label-free imaging of human brain cancer in a systematic study which compares brain cancer with noncancer tissues using freshly resected ex vivo human tissues, and in vivo murine model implanted with human gliomas (Figure 3) [76]. In addition, this study proposes a method to quantitatively analyze and display OCT imaging results with high sensitivity and specificity. Most importantly, a color-coded optical property map is generated in real time which provides direct visual cues in detecting brain cancer.
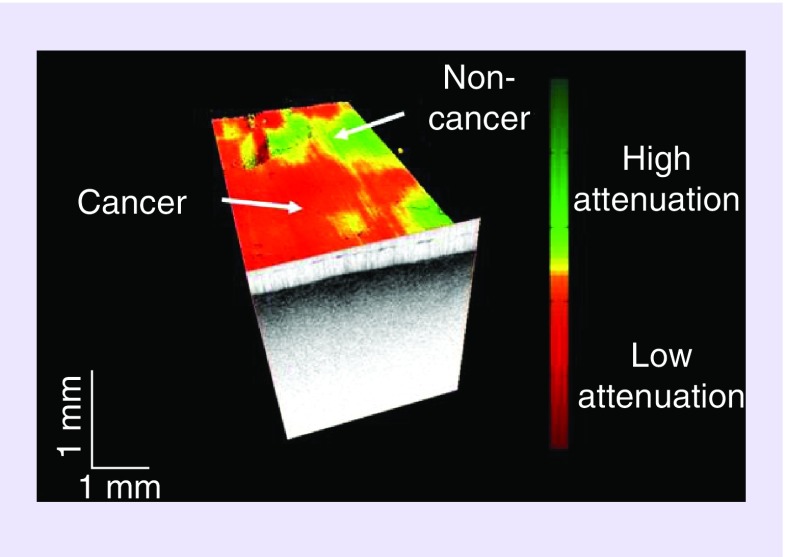
Figure 3. . OCT image detecting cancer from non-cancer brain tissue.
Optical Coherence Tomography can provide direct visual cues todistinguish glioma (red) from non-tumor (green). Here, a volumetric OCT dataset is shown with an overlaid color-coded optical property map which shows brain tumor versus non-tumor. The optical property map can be acquired, processed and displayed in real-time and without the need of any contrast agents for imaging.
Modified and reprinted with permission from Science Translational Medicine [76].
To enable real-time OCT imaging, the 2015 study utilized a home-built, swept-source OCT imaging system which operates at a central wavelength of 1300 nm and at a 3 dB spectral bandwidth of approximately 110–130 nm. On average, the laser source outputs about 15 mW to the brain tissue sample through the OCT handheld imaging probe. Using a high-speed data acquisition card and Graphics Processing Unit (GPU)-based signal processing, Swept Source (SS)-OCT images can be acquired, processed, displayed and stored in real time at a speed of up to 220,000 A-lines/s, or up to 220 frames/s (fps) assuming that each frame consists of approximately 1000 A-lines and approximately 2000 pixels per A-line. This translates to a total of 1.2–2.4 s for each 8–16 mm3 volumetric tissue block [76].
For most systemic tumors, the optical attenuation is generally higher in cancer (compared with noncancer) due to a higher cell density and nuclear-to-cytoplasmic ratio. In the human brain, however, brain cancer actually has a lower attenuation when comparing with the surrounding noncancer white matter. To fully understand why brain cancer actually has a lower attenuation than noncancer white matter, we need to first understand the biological and physical properties which determine the optical attenuation for a tissue sample. In brain cancer, optical attenuation is governed by several important factors. First of all, it is well known that noncancer white matter in the brain has a high attenuation due to its rich myelin content [68,74,76,77]. When brain cancer proliferates, it will ultimately induce the breakdown of myelin in white matter to facilitate infiltration into the surrounding normal brain [78–80]. Noncancer gray matter, on the other hand, does not contain myelin. As a result, brain cancer is found to have a lower overall attenuation when compared with noncancer white matter, but a higher overall attenuation when compared with noncancer gray matter [76].
Given our understanding of the aforementioned methodologies, the 2015 study investigated the potential of OCT in imaging brain cancer via a systematic study with 32 consented patients (15 patients with HGG, 12 patients with LGG and 5 control patients with noncancer brain lesions) and over 4600 optical attenuation data points [76]. First, a set of optical attenuation parameters were established to analyze a training set (with 16 patients) to establish a diagnostic attenuation threshold (for detection of brain cancer vs noncancer); then the final 16 patients were entered into a double-blinded study to compute the OCT detection [76]. In this study, an independent and double-blinded study was conducted to determine the sensitivity and specificity associated with the optimal attenuation threshold (at 5.5 mm-1). The OCT detection sensitivity was found to be 92% and specificity was determined to be at 100% for HGG patients (n = 7) [76]. Using the same diagnostic attenuation threshold, the sensitivity was found to be 100% and specificity was found to be 80% for LGG patients (n = 9) [76]. This was also confirmed with in vivo mouse models implanted with human GBM (Figure 4). Thus, OCT represents an experimental imaging technology which has been successfully applied in clinical settings, for example, ophthalmology, gastrointestinal and intravascular applications, and has demonstrated early promise in differentiating cancer from noncancer tissue based on ex vivo human and in vivo animal data.
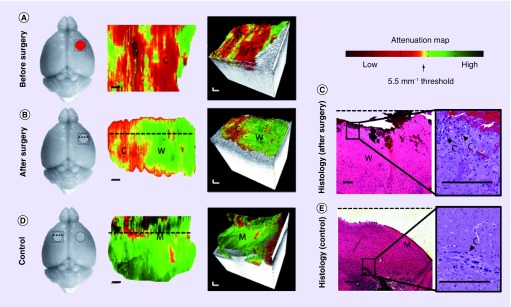
Figure 4. .
In vivo OCT imaging and optical property mapping in mice implanted with human glioma before (A) and after (B) surgery, and at normal brain surface (C). OCT results are confirmed with corresponding histology as shown in (D), which are 2D cross-sectional histological sections perpendicular to the optical attenuation map along the dotted lines.
Scale bars: 1 mm.
Reprinted with permission from Science Translational Medicine [76].
Conclusion
To summarize, maximizing the extent of brain cancer resection can prolong survival and delay recurrence. It is important to recognize that different technologies have different levels of resolution, sensitivity and specificity in detecting tumor tissue (Table 3). However, it is challenging to distinguish cancer from noncancer tissues intraoperatively, especially at the infiltrative margins. Thus, neurosurgeons are faced with the challenge of maximizing the removal of brain cancer while preserving surrounding normal brain (especially eloquent areas, e.g., motor, speech and sensory areas). This review described the use of both conventional (i.e., US, MRI and fluorescence-guided resections) as well as experimental imaging techniques (i.e., Raman and OCT) in imaging glioma surgery. Specifically, it provides details on the methodology, results and promise of different methodologies used to differentiate tumor from noncancer tissue in real time. Many of these technologies have been integrated into the neurosurgical operating room and other newer technologies have demonstrated effectiveness improving detection of tumor tissue. We believe that by combining some of these techniques, better results in terms of extent of resection, neurologic outcomes and survival of patients with gliomas will be achieved. Large-scale clinical trials are needed to truly assess the utility of the different intraoperative modalities in glioma surgery. As technology is incorporated into the neurosurgical operating room, more information and guidelines for its use will emerge. This will result in standardized use and maximization of the utility to provide optimal surgical treatment for brain cancer patients. Unfortunately, many of the studies reviewed are retrospective in nature and the data obtained from them have the inherent limitations of retrospective studies, highlighting the need for large randomized clinical trials to understand the usefulness of the different intraoperative imaging modalities in the care of glioma patients.
Table 3. . Comparison of different technologies in surgical guidance of brain cancer.
| Feature | Ultrasound | iMRI | 5-ALA fluorescence | Raman | OCT |
|---|---|---|---|---|---|
| Resolution | 0.3 mm3 | 3–20 mm3 | 0.001 mm2 | 0.00000025 mm2 | 0.004 mm3 |
| FOV | 12,500 mm3 | Whole brain | 75–2000 mm2 | 0.1225 mm3 | 8-16 mm3 |
| Continuous guidance? | Yes | No | Yes | Yes | Yes |
| 3D? | Yes | Yes | No | No | Yes |
| Numerical data? | No | No | Yes | Yes | Yes |
| Sensitivity (during and/or post-resection) (%) | 26–87 | N/A | 47 (visual) 84 (spectrometry) |
N/A (accuracy 99–100) | 92–100 |
| Specificity (during and/or post-resection) (%) | 42–88 | N/A | 100 (visual) 92 (spectrometry) |
N/A | 80–100 |
Various technological advances have attempted to increase the surgeon's ability to identify cancer tissue. Each modality has different strengths and limitations in terms of resolution, FOV, sensitivity/specificity and the ability to provide quantitative and 3D continuous imaging guidance. Modified and reprinted with permission from Science Translational Medicine [76].
FOV: Field of view; OCT: Optical coherence tomography.
Future perspective
With the evolution of more sophisticated technologies and the potential of implementation in the neurosurgical operating room, the sensitivity and accuracy to detect glioma tissue during surgery will increase significantly. Through technological advances, the intraoperative technologies discussed in this manuscript could potentially be merged and used seamlessly in the operating room to spatially differentiate cancer from noncancer within the resection cavity and to allow seamless and continuous surgical guidance in the operating room. Future large-scale clinical trials are required to obtain better evidence and identify better ways to employ intraoperative imaging in the operating room. With additional clinical studies and technological advances, we are confident that intraoperative imaging techniques will become a very important part of the surgical technique for brain tumor resection, which will ultimately result in cleaner resection margins for glioma patients and thus prolonged overall survival and delayed recurrence for patients.
EXECUTIVE SUMMARY.
Intraoperative ultrasound
Intraoperative ultrasound (IOUS) is a widely available and inexpensive tool.
IOUS can differentiate normal tissue from neoplastic tissue improving resection in a large percentage of patients.
Limitations of IOUS include user-dependent limitations, image quality, presence of edema and hemorrhage within tumors and absence of functional data.
Intraoperative MRI
Intraoperative MRI (iMRI) can bypass the effects of brain shift and improve the accuracy of neuronavigation.
iMRI can detect residual tissue accurately and improve the extent of resection.
Limitations include its low prevalence throughout neurosurgical centers and cost of implementation.
Fluorescence-guided microsurgery
Fluorescence-guided microsurgery is an inexpensive technique that can be combined with iMRI and IOUS to improve resection of gliomas.
Fluorescent agents used are not currently US FDA approved for their use in glioma surgery in the US.
Limitations include availability of microscopes with the necessary optics for implementation, skin photosensitivity, hypotension and the limitation of its use in color blind surgeons.
Raman spectroscopy
New experimental imaging technology with promising pilot results.
Raman spectroscopy characterizes tissue based on molecular fingerprint for tumor versus nontumor.
Optical coherence tomography
FDA approved with successful clinical applications in ophthalmology, gastroenterology and cardiology.
Experimental technique used to visualize brain tissues noninvasively and at micron-level resolution.
Optical coherence tomography characterizes brain tissues based on optical attenuation differences in tumor versus nontumor.
Color-coded optical property map can be generated in real time to provide direct visual cues in detecting brain cancer.
Ex vivo human and in vivo animal studies show promise; additional in vivo clinical studies are needed to evaluate feasibility of this approach.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J. Neurosurg. 2001;95(2):190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 2.Lote K, Egeland T, Hager B, et al. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J. Clin. Oncol. 1997;15(9):3129–3140. doi: 10.1200/JCO.1997.15.9.3129. [DOI] [PubMed] [Google Scholar]
- 3.Mcgirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63(4):700–707. doi: 10.1227/01.NEU.0000325729.41085.73. author reply 707–708. [DOI] [PubMed] [Google Scholar]
- 4.Mcgirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J. Neurosurg. 2009;110(1):156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 5.Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16(1):113–122. doi: 10.1093/neuonc/not137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura M, Konishi N, Tsunoda S, et al. Analysis of prognostic and survival factors related to treatment of low-grade astrocytomas in adults. Oncology. 2000;58(2):108–116. doi: 10.1159/000012087. [DOI] [PubMed] [Google Scholar]
- 7.Dohrmann GJ, Rubin JM. History of intraoperative ultrasound in neurosurgery. Neurosurg. Clin. N. Am. 2001;12(1):155–166. ix. [PubMed] [Google Scholar]
- 8.Moiyadi AV. Intraoperative ultrasound technology in neuro-oncology practice-current role and future applications. World Neurosurg. 2016;93:81–93. doi: 10.1016/j.wneu.2016.05.083. [DOI] [PubMed] [Google Scholar]
- 9.Christian E, Yu C, Apuzzo ML. Focused ultrasound: relevant history and prospects for the addition of mechanical energy to the neurosurgical armamentarium. World Neurosurg. 2014;82(3–4):354–365. doi: 10.1016/j.wneu.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Almeida JP, Chaichana KL, Rincon-Torroella J, Quinones-Hinojosa A. The value of extent of resection of glioblastomas: clinical evidence and current approach. Curr. Neurol. Neurosci. Rep. 2015;15(2):517. doi: 10.1007/s11910-014-0517-x. [DOI] [PubMed] [Google Scholar]
- 11.Leroux PD, Winter TC, Berger MS, Mack LA, Wang K, Elliott JP. A comparison between preoperative magnetic resonance and intraoperative ultrasound tumor volumes and margins. J. Clin. Ultrasound. 1994;22(1):29–36. doi: 10.1002/jcu.1870220107. [DOI] [PubMed] [Google Scholar]
- 12.Solheim O, Selbekk T, Jakola AS, Unsgard G. Ultrasound-guided operations in unselected high-grade gliomas – overall results, impact of image quality and patient selection. Acta Neurochir. (Wien.) 2010;152(11):1873–1886. doi: 10.1007/s00701-010-0731-5. [DOI] [PubMed] [Google Scholar]
- 13.Unsgard G, Solheim O, Lindseth F, Selbekk T. Intra-operative imaging with 3D ultrasound in neurosurgery. Acta Neurochir. Suppl. 2011;109:181–186. doi: 10.1007/978-3-211-99651-5_28. [DOI] [PubMed] [Google Scholar]
- 14.Woydt M, Krone A, Becker G, Schmidt K, Roggendorf W, Roosen K. Correlation of intra-operative ultrasound with histopathologic findings after tumour resection in supratentorial gliomas. A method to improve gross total tumour resection. Acta Neurochir. (Wien.) 1996;138(12):1391–1398. doi: 10.1007/BF01411117. [DOI] [PubMed] [Google Scholar]
- 15.Rygh OM, Selbekk T, Torp SH, Lydersen S, Hernes TA, Unsgaard G. Comparison of navigated 3D ultrasound findings with histopathology in subsequent phases of glioblastoma resection. Acta Neurochir. (Wien.) 2008;150(10):1033–1041. doi: 10.1007/s00701-008-0017-3. discussion 1042. [DOI] [PubMed] [Google Scholar]
- 16.Selbekk T, Jakola AS, Solheim O, et al. Ultrasound imaging in neurosurgery: approaches to minimize surgically induced image artefacts for improved resection control. Acta Neurochir. (Wien.) 2013;155(6):973–980. doi: 10.1007/s00701-013-1647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moiyadi AV, Shetty PM, Mahajan A, Udare A, Sridhar E. Usefulness of three-dimensional navigable intraoperative ultrasound in resection of brain tumors with a special emphasis on malignant gliomas. Acta Neurochir. (Wien.) 2013;155(12):2217–2225. doi: 10.1007/s00701-013-1881-z. [DOI] [PubMed] [Google Scholar]
- 18.Le Roux PD, Berger MS, Wang K, Mack LA, Ojemann GA. Low grade gliomas: comparison of intraoperative ultrasound characteristics with preoperative imaging studies. J. Neurooncol. 1992;13(2):189–198. doi: 10.1007/BF00172770. [DOI] [PubMed] [Google Scholar]
- 19.Chacko AG, Kumar NK, Chacko G, Athyal R, Rajshekhar V. Intraoperative ultrasound in determining the extent of resection of parenchymal brain tumours – a comparative study with computed tomography and histopathology. Acta Neurochir. (Wien.) 2003;145(9):743–748. doi: 10.1007/s00701-003-0009-2. discussion 748. [DOI] [PubMed] [Google Scholar]
- 20.Erdogan N, Tucer B, Mavili E, Menku A, Kurtsoy A. Ultrasound guidance in intracranial tumor resection: correlation with postoperative magnetic resonance findings. Acta Radiol. 2005;46(7):743–749. doi: 10.1080/02841850500223208. [DOI] [PubMed] [Google Scholar]
- 21.Hammoud MA, Ligon BL, Elsouki R, Shi WM, Schomer DF, Sawaya R. Use of intraoperative ultrasound for localizing tumors and determining the extent of resection: a comparative study with magnetic resonance imaging. J. Neurosurg. 1996;84(5):737–741. doi: 10.3171/jns.1996.84.5.0737. [DOI] [PubMed] [Google Scholar]
- 22.Mari AR, Shah I, Imran M, Ashraf J. Role of intraoperative ultrasound in achieving complete resection of intra-axial solid brain tumours. J. Pak. Med. Assoc. 2014;64(12):1343–1347. [PubMed] [Google Scholar]
- 23.Renovanz M, Hickmann AK, Henkel C, Nadji-Ohl M, Hopf NJ. Navigated versus non-navigated intraoperative ultrasound: is there any impact on the extent of resection of high-grade gliomas? A retrospective clinical analysis. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2014;75(3):224–230. doi: 10.1055/s-0033-1356486. [DOI] [PubMed] [Google Scholar]
- 24.Serra C, Stauffer A, Actor B, et al. Intraoperative high frequency ultrasound in intracerebral high-grade tumors. Ultraschall Med. 2012;33(7):E306–E312. doi: 10.1055/s-0032-1325369. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Duan YY, Liu X, et al. Application of intraoperative ultrasonography for guiding microneurosurgical resection of small subcortical lesions. Korean J. Radiol. 2011;12(5):541–546. doi: 10.3348/kjr.2011.12.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahboob S, McPhillips R, Qiu Z, et al. Intraoperative ultrasound-guided resection of gliomas: a meta-analysis and review of the literature. World Neurosurg. 2016;92:255–263. doi: 10.1016/j.wneu.2016.05.007. [DOI] [PubMed] [Google Scholar]; • In this extensive meta-analysis of current literature it was found that ultrasound-guided surgical resection of gliomas improves the extent of resection.
- 27.Moiyadi AV, Kannan S, Shetty P. Navigated intraoperative ultrasound for resection of gliomas: predictive value, influence on resection and survival. Neurol. India. 2015;63(5):727–735. doi: 10.4103/0028-3886.166549. [DOI] [PubMed] [Google Scholar]
- 28.Saether CA, Torsteinsen M, Torp SH, Sundstrom S, Unsgard G, Solheim O. Did survival improve after the implementation of intraoperative neuronavigation and 3D ultrasound in glioblastoma surgery? A retrospective analysis of 192 primary operations. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2012;73(2):73–78. doi: 10.1055/s-0031-1297247. [DOI] [PubMed] [Google Scholar]
- 29.Jakola AS, Unsgard G, Solheim O. Quality of life in patients with intracranial gliomas: the impact of modern image-guided surgery. J. Neurosurg. 2011;114(6):1622–1630. doi: 10.3171/2011.1.JNS101657. [DOI] [PubMed] [Google Scholar]
- 30.Prada F, Bene MD, Fornaro R, et al. Identification of residual tumor with intraoperative contrast-enhanced ultrasound during glioblastoma resection. Neurosurg. Focus. 2016;40(3):E7. doi: 10.3171/2015.11.FOCUS15573. [DOI] [PubMed] [Google Scholar]
- 31.Mattei L, Prada F, Legnani FG, Perin A, Olivi A, Dimeco F. Neurosurgical tools to extend tumor resection in hemispheric low-grade gliomas: conventional and contrast enhanced ultrasonography. Childs Nerv. Syst. 2016;32(10):1907–1914. doi: 10.1007/s00381-016-3186-z. [DOI] [PubMed] [Google Scholar]
- 32.Sastry R, Bi WL, Pieper S, et al. Applications of ultrasound in the resection of brain tumors. J. Neuroimaging. 2017;27(1):5–15. doi: 10.1111/jon.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black PM, Moriarty T, 3rd, et al. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery. 1997;41(4):831–842. doi: 10.1097/00006123-199710000-00013. discussion 842–835. [DOI] [PubMed] [Google Scholar]
- 34.Kubben PL, ter Meulen KJ, Schijns OE, ter Laak-Poort MP, van Overbeeke JJ, van Santbrink H. Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol. 2011;12(11):1062–1070. doi: 10.1016/S1470-2045(11)70130-9. [DOI] [PubMed] [Google Scholar]
- 35.Schneider JP, Trantakis C, Rubach M, et al. Intraoperative MRI to guide the resection of primary supratentorial glioblastoma multiforme – a quantitative radiological analysis. Neuroradiology. 2005;47(7):489–500. doi: 10.1007/s00234-005-1397-1. [DOI] [PubMed] [Google Scholar]
- 36.Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011;12(11):997–1003. doi: 10.1016/S1470-2045(11)70196-6. [DOI] [PubMed] [Google Scholar]; • To date this is the only randomized, controlled trial evaluating the role of intraoperative MRI in glioma surgery and showing that it improves extent of resection.
- 37.Coburger J, Wirtz CR, Konig RW. Impact of extent of resection and recurrent surgery on clinical outcome and overall survival in a consecutive series of 170 patients for glioblastoma in intraoperative high field iMRI. J. Neurosurg. Sci. 2015;61(3):233–244. doi: 10.23736/S0390-5616.16.03284-7. [DOI] [PubMed] [Google Scholar]
- 38.Kubben PL, Scholtes F, Schijns OE, et al. Intraoperative magnetic resonance imaging versus standard neuronavigation for the neurosurgical treatment of glioblastoma: a randomized controlled trial. Surg. Neurol. Int. 2014;5:70. doi: 10.4103/2152-7806.132572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eljamel MS, Mahboob SO. The effectiveness and cost-effectiveness of intraoperative imaging in high-grade glioma resection; a comparative review of intraoperative ALA, fluorescein, ultrasound and MRI. Photodiagnosis Photodyn. Ther. 2016;16:35–43. doi: 10.1016/j.pdpdt.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Coburger J, Scheuerle A, Thal DR, et al. Linear array ultrasound in low-grade glioma surgery: histology-based assessment of accuracy in comparison to conventional intraoperative ultrasound and intraoperative MRI. Acta Neurochir. (Wien.) 2015;157(2):195–206. doi: 10.1007/s00701-014-2314-3. [DOI] [PubMed] [Google Scholar]
- 41.Coburger J, Hagel V, Wirtz CR, Konig R. Surgery for glioblastoma: impact of the combined use of 5-aminolevulinic acid and intraoperative MRI on extent of resection and survival. PLoS ONE. 2015;10(6):e0131872. doi: 10.1371/journal.pone.0131872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schatlo B, Fandino J, Smoll NR, et al. Outcomes after combined use of intraoperative MRI and 5-aminolevulinic acid in high-grade glioma surgery. Neuro Oncol. 2015;17(12):1560–1567. doi: 10.1093/neuonc/nov049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foroglou N, Zamani A, Black P. Intra-operative MRI (iop-MR) for brain tumour surgery. Br. J. Neurosurg. 2009;23(1):14–22. doi: 10.1080/02688690802610587. [DOI] [PubMed] [Google Scholar]
- 44.Kubben PL, Wesseling P, Lammens M, et al. Correlation between contrast enhancement on intraoperative magnetic resonance imaging and histopathology in glioblastoma. Surg. Neurol. Int. 2012;3:158. doi: 10.4103/2152-7806.105097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez L, Batlle A, Di Venosa G, et al. Study of the mechanisms of uptake of 5-aminolevulinic acid derivatives by PEPT1 and PEPT2 transporters as a tool to improve photodynamic therapy of tumours. Int. J. Biochem. Cell Biol. 2006;38(9):1530–1539. doi: 10.1016/j.biocel.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Ishikawa T, Takahashi K, Ikeda N, et al. Transporter-mediated drug interaction strategy for 5-aminolevulinic acid (ALA)-based photodynamic diagnosis of malignant brain tumor: molecular design of ABCG2 inhibitors. Pharmaceutics. 2011;3(3):615–635. doi: 10.3390/pharmaceutics3030615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre Phase III trial. Lancet Oncol. 2006;7(5):392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 48.Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J. Neurosurg. 2000;93(6):1003–1013. doi: 10.3171/jns.2000.93.6.1003. [DOI] [PubMed] [Google Scholar]
- 49.Eljamel S. 5-ALA fluorescence image guided resection of glioblastoma multiforme: a meta-analysis of the literature. Int. J. Mol. Sci. 2015;16(5):10443–10456. doi: 10.3390/ijms160510443. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is a systematic meta-analysis of multiple studies evaluating the effectiveness of 5-aminolevulinic acid in increasing extent of resection in glioma surgery. The authors showed that this technique increases extent of resection as compared with not using it.
- 50.Díez Valle R, Tejada Solis S, Idoate Gastearena MA, Garcia De Eulate R, Dominguez Echavarri P, Aristu Mendiroz J. Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: volumetric analysis of extent of resection in single-center experience. J. Neurooncol. 2011;102(1):105–113. doi: 10.1007/s11060-010-0296-4. [DOI] [PubMed] [Google Scholar]
- 51.Hefti M, Von Campe G, Moschopulos M, Siegner A, Looser H, Landolt H. 5-aminolevulinic acid induced protoporphyrin IX fluorescence in high-grade glioma surgery: a one-year experience at a single institutuion. Swiss Med. Wkly. 2008;138(11–12):180–185. doi: 10.4414/smw.2008.12077. [DOI] [PubMed] [Google Scholar]
- 52.Roder C, Bisdas S, Ebner FH, et al. Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: high-field iMRI versus conventional and 5-ALA-assisted surgery. Eur. J. Surg. Oncol. 2014;40(3):297–304. doi: 10.1016/j.ejso.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 53.Yamada S, Muragaki Y, Maruyama T, Komori T, Okada Y. Role of neurochemical navigation with 5-aminolevulinic acid during intraoperative MRI-guided resection of intracranial malignant gliomas. Clin. Neurol. Neurosurg. 2015;130:134–139. doi: 10.1016/j.clineuro.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Zhao S, Wu J, Wang C, et al. Intraoperative fluorescence-guided resection of high-grade malignant gliomas using 5-aminolevulinic acid-induced porphyrins: a systematic review and meta-analysis of prospective studies. PLoS ONE. 2013;8(5):e63682. doi: 10.1371/journal.pone.0063682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuroiwa T, Kajimoto Y, Ohta T. Comparison between operative findings on malignant glioma by a fluorescein surgical microscopy and histological findings. Neurol. Res. 1999;21(1):130–134. doi: 10.1080/01616412.1999.11740909. [DOI] [PubMed] [Google Scholar]
- 56.Acerbi F, Broggi M, Eoli M, et al. Is fluorescein-guided technique able to help in resection of high-grade gliomas? Neurosurg. Focus. 2014;36(2):E5. doi: 10.3171/2013.11.FOCUS13487. [DOI] [PubMed] [Google Scholar]
- 57.Schebesch KM, Proescholdt M, Hohne J, et al. Sodium fluorescein-guided resection under the YELLOW 560 nm surgical microscope filter in malignant brain tumor surgery – a feasibility study. Acta Neurochir. (Wien.) 2013;155(4):693–699. doi: 10.1007/s00701-013-1643-y. [DOI] [PubMed] [Google Scholar]
- 58.Koc K, Anik I, Cabuk B, Ceylan S. Fluorescein sodium-guided surgery in glioblastoma multiforme: a prospective evaluation. Br. J. Neurosurg. 2008;22(1):99–103. doi: 10.1080/02688690701765524. [DOI] [PubMed] [Google Scholar]
- 59.Neira JA, Ung TH, Sims JS, et al. Aggressive resection at the infiltrative margins of glioblastoma facilitated by intraoperative fluorescein guidance. J. Neurosurg. 2016:1–12. doi: 10.3171/2016.7.JNS16232. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 60.Kairdolf BA, Bouras A, Kaluzova M, et al. Intraoperative spectroscopy with ultrahigh sensitivity for image-guided surgery of malignant brain tumors. Anal. Chem. 2016;88(1):858–867. doi: 10.1021/acs.analchem.5b03453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung IW, Eljamel S. Risk factors for developing oral 5-aminolevulinic acid-induced side effects in patients undergoing fluorescence guided resection. Photodiagnosis Photodyn. Ther. 2013;10(4):362–367. doi: 10.1016/j.pdpdt.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 62.Petterssen M, Eljamel S, Eljamel S. Protoporphyrin-IX fluorescence guided surgical resection in high-grade gliomas: the potential impact of human colour perception. Photodiagnosis Photodyn. Ther. 2014;11(3):351–356. doi: 10.1016/j.pdpdt.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 63.Schucht P, Seidel K, Beck J, et al. Intraoperative monopolar mapping during 5-ALA-guided resections of glioblastomas adjacent to motor eloquent areas: evaluation of resection rates and neurological outcome. Neurosurg. Focus. 2014;37(6):E16. doi: 10.3171/2014.10.FOCUS14524. [DOI] [PubMed] [Google Scholar]
- 64.Jermyn M, Mok K, Mercier J, et al. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci. Transl. Med. 2015;7(274):274ra219. doi: 10.1126/scitranslmed.aaa2384. [DOI] [PubMed] [Google Scholar]; • This is a recent paper which demonstrated exciting intraoperative results for Raman spectroscopy, which is able to detect normal brain from cancer-infiltrated areas in 17 glioma patients with a high sensitivity and specificity.
- 65.Ji M, Orringer DA, Freudiger CW, et al. Rapid, label-free detection of brain tumors with stimulated raman scattering microscopy. Sci. Transl. Med. 2013;5(201):201ra119. doi: 10.1126/scitranslmed.3005954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat. Biotechnol. 2003;21(11):1361–1367. doi: 10.1038/nbt892. [DOI] [PubMed] [Google Scholar]
- 67.Boppart SA, Brezinski ME, Pitris C, Fujimoto JG. Optical coherence tomography for neurosurgical imaging of human intracortical melanoma. Neurosurgery. 1998;43(4):834–841. doi: 10.1097/00006123-199810000-00068. [DOI] [PubMed] [Google Scholar]
- 68.Böhringer H, Boller D, Leppert J, et al. Time-domain and spectral-domain optical coherence tomography in the analysis of brain tumor tissue. Lasers Surg. Med. 2006;38(6):588–597. doi: 10.1002/lsm.20353. [DOI] [PubMed] [Google Scholar]; • This is the first optical coherence tomography study showing in vivo, 2D cross-sectional imaging of high-grade gliomas in patients with attenuation data.
- 69.Kantelhardt S, Finke M, Schweikard A, Giese A. Evaluation of a completely robotized neurosurgical operating microscope. Neurosurgery. 2013;72:A19–A26. doi: 10.1227/NEU.0b013e31827235f8. [DOI] [PubMed] [Google Scholar]
- 70.Finke M, Kantelhardt S, Schlaefer A, et al. Automatic scanning of large tissue areas in neurosurgery using optical coherence tomography. Int. J. Med. Robot. 2012;8(3):327–336. doi: 10.1002/rcs.1425. [DOI] [PubMed] [Google Scholar]
- 71.Bohringer HJ, Lankenau E, Stellmacher F, Reusche E, Huttmann G, Giese A. Imaging of human brain tumor tissue by near-infrared laser coherence tomography. Acta Neurochir. (Wien.) 2009;151(5):507–517. doi: 10.1007/s00701-009-0248-y. discussion 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bizheva K, Drexler W, Preusser M, et al. Imaging ex vivo healthy and pathological human brain tissue with ultra-high-resolution optical coherence tomography. J. Biomed. Opt. 2005;10(1):11006. doi: 10.1117/1.1851513. [DOI] [PubMed] [Google Scholar]
- 73.Levitz D, Thrane L, Frosz M, et al. Determination of optical scattering properties of highly-scattering media in optical coherence tomography images. Opt. Express. 2004;12(2):249–259. doi: 10.1364/opex.12.000249. [DOI] [PubMed] [Google Scholar]
- 74.Assayag O, Grieve K, Devaux B, et al. Imaging of non-tumorous and tumorous human brain tissue with full-field optical coherence tomography. NeuroImage Clin. 2013 doi: 10.1016/j.nicl.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bohringer HJ, Lankenau E, Rohde V, Huttmann G, Giese A. Optical coherence tomography for experimental neuroendoscopy. Minim. Invasive Neurosurg. 2006;49(5):269–275. doi: 10.1055/s-2006-954574. [DOI] [PubMed] [Google Scholar]
- 76.Kut C, Chaichana KL, Raza SM, et al. Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci. Transl. Med. 2015;7(292):292ra100. doi: 10.1126/scitranslmed.3010611. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is the first systematic quantitative optical coherence tomography study for brain cancer in 32 patients which established a diagnostic attenuation threshold capable of differentiating tumor versus nontumor tissues with high sensitivity/specificity, and to display intuitive cues for detection of brain cancer margins using color-coded optical property maps.
- 77.Cobb MJ, Chen Y, Bailey SL, Kemp CJ, Li XD. Non-invasive imaging of carcinogen-induced early neoplasia using ultrahigh-resolution optical coherence tomography. Cancer Biomark. 2006;2(3–4):163–173. doi: 10.3233/cbm-2006-23-408. [DOI] [PubMed] [Google Scholar]
- 78.Alaminos M, Dávalos V, Ropero S, et al. EMP3, a myelin-related gene located in the critical 19q13. 3 region, is epigenetically silenced and exhibits features of a candidate tumor suppressor in glioma and neuroblastoma. Cancer Res. 2005;65(7):2565–2571. doi: 10.1158/0008-5472.CAN-04-4283. [DOI] [PubMed] [Google Scholar]
- 79.Amberger VR, Hensel T, Ogata N, Schwab ME. Spreading and migration of human glioma and rat C6 cells on central nervous system myelin in vitro is correlated with tumor malignancy and involves a metalloproteolytic activity. Cancer Res. 1998;58(1):149–158. [PubMed] [Google Scholar]
- 80.Binder DK, Berger MS. Proteases and the biology of glioma invasion. J. Neurooncol. 2002;56(2):149–158. doi: 10.1023/a:1014566604005. [DOI] [PubMed] [Google Scholar]