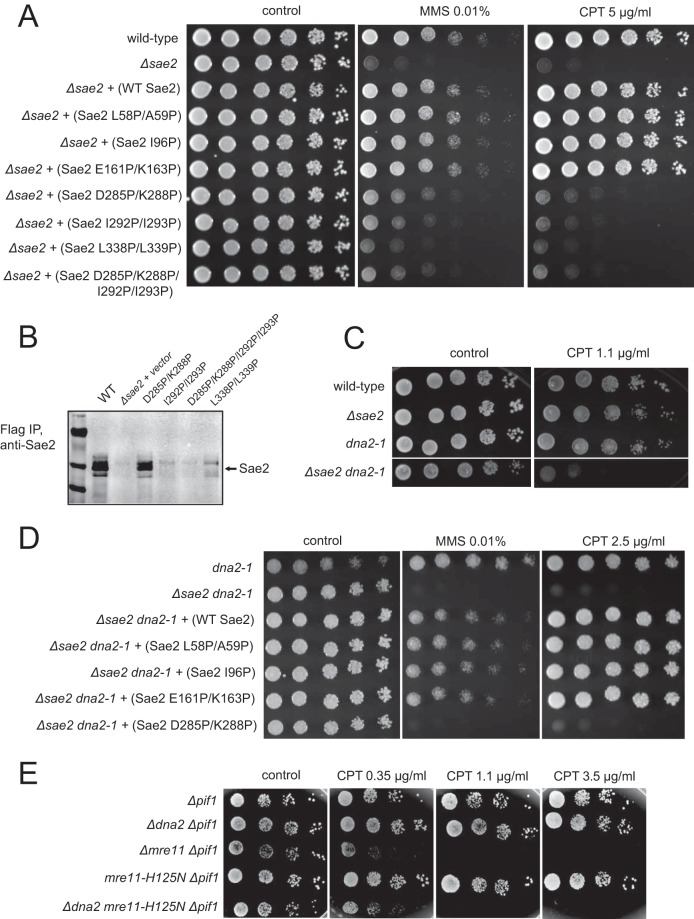
FIG 2.
Mutation of helix-nucleating segments increases the sensitivity of Sae2-deficient yeast cells to DNA damage. (A) FLAG-tagged Sae2 was expressed from a CEN plasmid under the control of the native Sae2 promoter in sae2Δ yeast cells. Fivefold serial dilutions of cells expressing the indicated Sae2 alleles were plated onto nonselective medium (control) or medium containing methyl methanesulfonate (MMS) (0.01%) or camptothecin (CPT) (5.0 μg/ml) and grown for 48 h. WT, wild type. (B) FLAG-tagged Sae2 C-terminal mutants were expressed from a 2μ plasmid in sae2Δ yeast cells and isolated by immunoprecipitation (IP) with anti-FLAG antibody. Sae2 levels in the immunoprecipitates were determined by Western blotting with anti-FLAG antibody. (C) sae2Δ yeast cells were compared to a strain with the nuclease-deficient Dna2 allele dna2-1 or a Δsae2 dna2-1 double mutant using serial dilutions of the cells as described above for panel A. (D) The dna2-1 or sae2Δ dna2-1 yeast strain complemented with FLAG-tagged Sae2 alleles expressed from a CEN plasmid under the control of the native Sae2 promoter, as indicated, was tested for MMS and CPT sensitivity as described above for panel A. Plates with DNA-damaging agents were incubated for 90 h, while the control plate was incubated for 70 h. (E) Δpif1, Δdna2 Δpif1, Δmre11 Δpif1, mre11-H125N Δpif1, and Δdna2 mre11-H125N Δpif1 strains were analyzed for CPT sensitivity as described above for panel A.