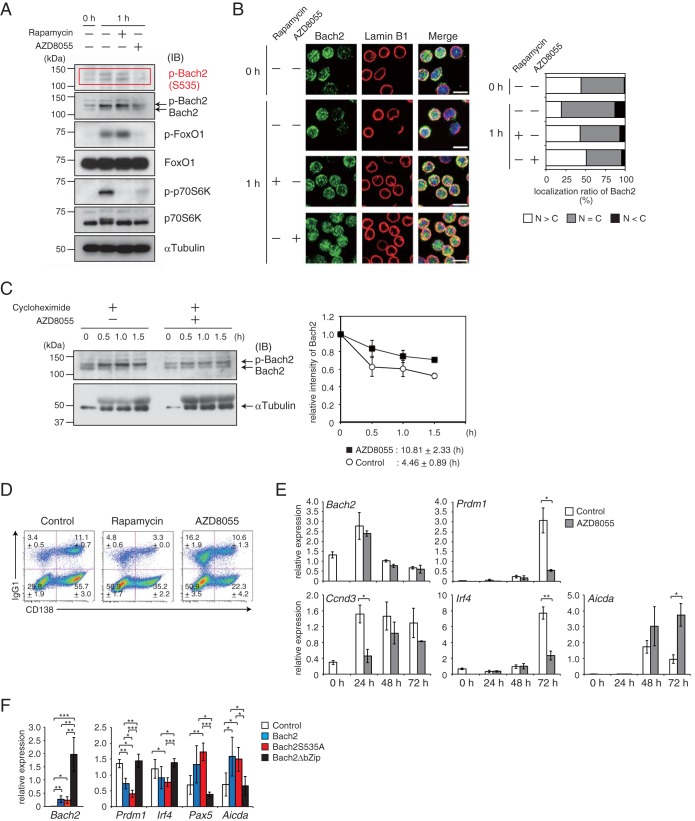
FIG 7.
Decision of CSR and PC differentiation via the mTORC-Bach2 cascade. Splenic B cells were isolated from B1-8hi mice and stimulated with IL-2, IL-4, IL-5, CD40L, and NP-Ficoll. Cells were cultured for 1 h in the presence of rapamycin or AZD8055 (A and B). (A) Immunoblot analysis for phosphorylated Bach2 at S535 (p-Bach2), total Bach2, phosphorylated FoxO1 (p-FoxO1), FoxO1, p-p70S6K, and p70S6K. α-Tubulin served as an internal control. (B) Immunohistochemistry for Bach2 protein. (Left) Bach2 (green) and lamin B1 (red) distribution in cells. (Right) The subcellular localization of Bach2 was evaluated by classification of 100 cells as described for Fig. 1C. Bar, 10 μm. (C) Half-life of Bach2 protein. Cells were cultured with or without AZD8055 in the presence of cycloheximide under IL-7 Hi conditions. (Left) Immunoblot analysis of Bach2. (Right) Relative intensity of total Bach2 protein. The amounts of protein were normalized with α-tubulin. Data are from one experiment using three mice and are shown as means and SEM. (D) Flow-cytometric analysis of CD138 and IgG1. Data are representative of three mice analyzed in one experiment and are shown as the means and SEM. (E) Quantitative RT-PCR analysis of Bach2, Prdm1, Ccnd3, Irf4, and Aicda for the indicated time points in the presence of AZD. One experiment was performed using three mice. (F) Quantitative RT-PCR analysis of Bach2, Prdm1, Irf4, Pax5, and Aicda in cells transduced with control, WT Bach2, Bach2 S535A, or Bach2ΔbZip expression vectors and sorted based on GFP expression. One experiment was performed using four mice. For panels A and C, α-tubulin served as an internal control. IB, immunoblot. For panels D and F, relative expression was normalized by Β2M, and the means and SEM were from three (E) or four (F) mouse samples. *, P < 0.05; **, P < 0.01; ***, P < 0.001.