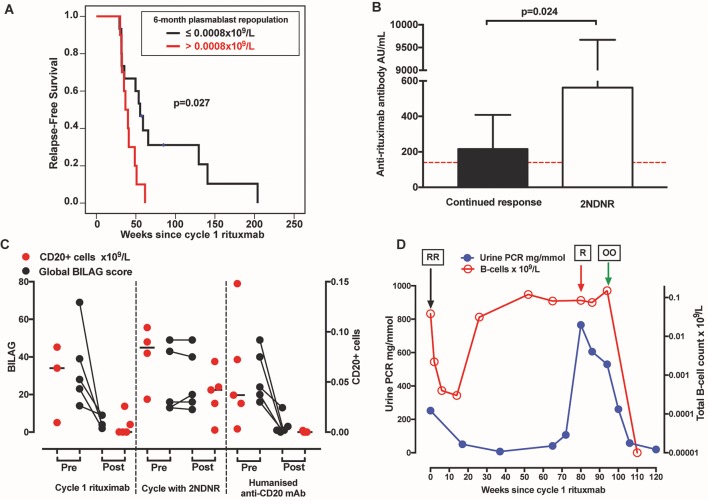
Figure 2.
2NDNR to rituximab and efficacy of alternative humanised anti-CD20 antibodies. (A) In this validation cohort, detection of plasmablasts >0.0008×109/L at 6 months predicted earlier relapse. (B) The phenomenon 2NDNR was associated with anti-rituximab antibody. The dotted red line represents normal cut-off of the test. (C) The Global BILAG score and CD20+ B-cells are plotted for each patient. The black line in the CD20+ B-cells figure represents the median. (D) An example of a case where proteinuria was normalised following a switch to ocrelizumab. ‘RR’ represents 2x infusions of rituximab, ‘R’ represents a single infusion as the patient cannot not complete the second due to severe infusion reaction and ‘OO’ represents 2x infusions of ocrelizumab. The total B-cell counts were transformed to natural log. 2NDNR, secondary non-depletion non-response; BILAG, British Isles Lupus Assessment Group.