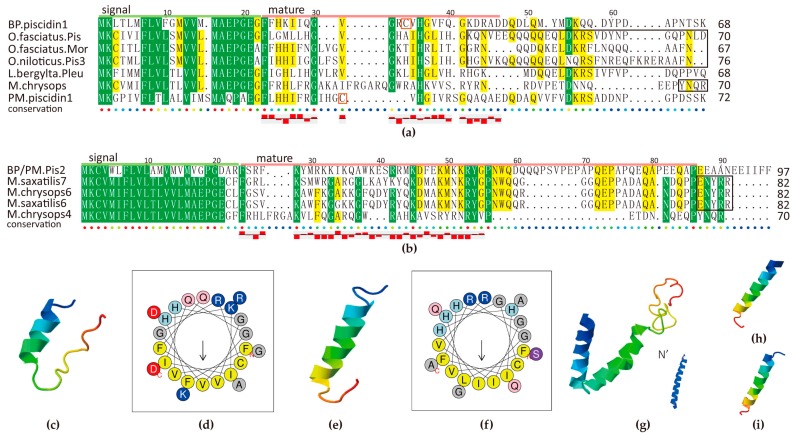
Figure 7.
Multiple sequence alignment of piscidin1 (a) and piscidin2 (b) and related structure analysis of mature peptides (c–i). The well-known prodomains are outlined in the boxes of (a,b). Yellow and green blocks indicate the areas with sequence identity >50% and >80% respectively. 3D structure predictions of BP piscidin1 (c), PM piscidin1 (e), piscidin2 and its N terminal (g), misgurin1 (h) and misgurin2 (i) all possess a α-helix. Helical wheel diagrams of BP piscidin1 (d) and PM piscidin1 (f) suggest an amphipathic nature by the alignment of the hydrophobic residues along one side of the helix and the other side of hydrophilic residues.