Fig. 2.
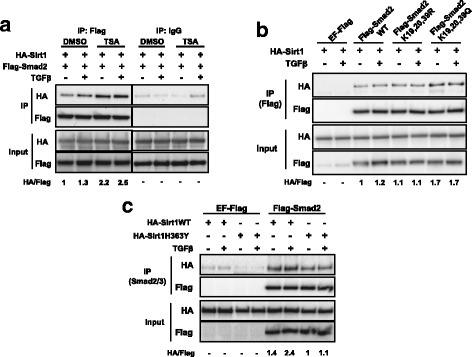
The interaction between Sirt1 and Smad2 is enhanced by Smad2 acetylation. a HEK293T cells transfected with the indicated plasmids were treated with SB-431542 and TSA or DMSO during 14 h, then washed out and treated with TGFβ for 1 h. Flag-Smad2 was immunoprecipitated and Smad2 and Sirt1 were detected by Western Blot. HA/Flag fold increase is indicated (lane 1–4 over lane 1). Weak Sirt1 binding to control samples were taken into account for calculations. The vertical lines across the blot indicate that two distant parts of the very same blot were put together. b HEK293T cells transfected with the indicated plasmids were treated with SB-431542 overnight, then washed out and treated with for 1 h. Flag proteins were immunoprecipitated and Sirt1 or Smad2WT and mutants were detected by Western Blot. HA/Flag fold increase is indicated (lane 3–8 over lane 3). c Hep3B cells transfected with the indicated plasmids were serum starved overnight and treated with TSA for 8 h followed by TGFβ for 1 h. Smad2/3 was purified by immunoprecipitation. Sirt1WT, Sirt1H363Y and Smad2 were detected by Western Blot. HA/Flag fold increase is indicated (lane 5–8 over lane 7). Weak Sirt1 binding to control samples were taken into account for calculations. All lanes are numbered from left to right. IP: immunoprecipitation, WT: wild type. All the experiments of this figure were repeated at least three times. Representative results are shown
