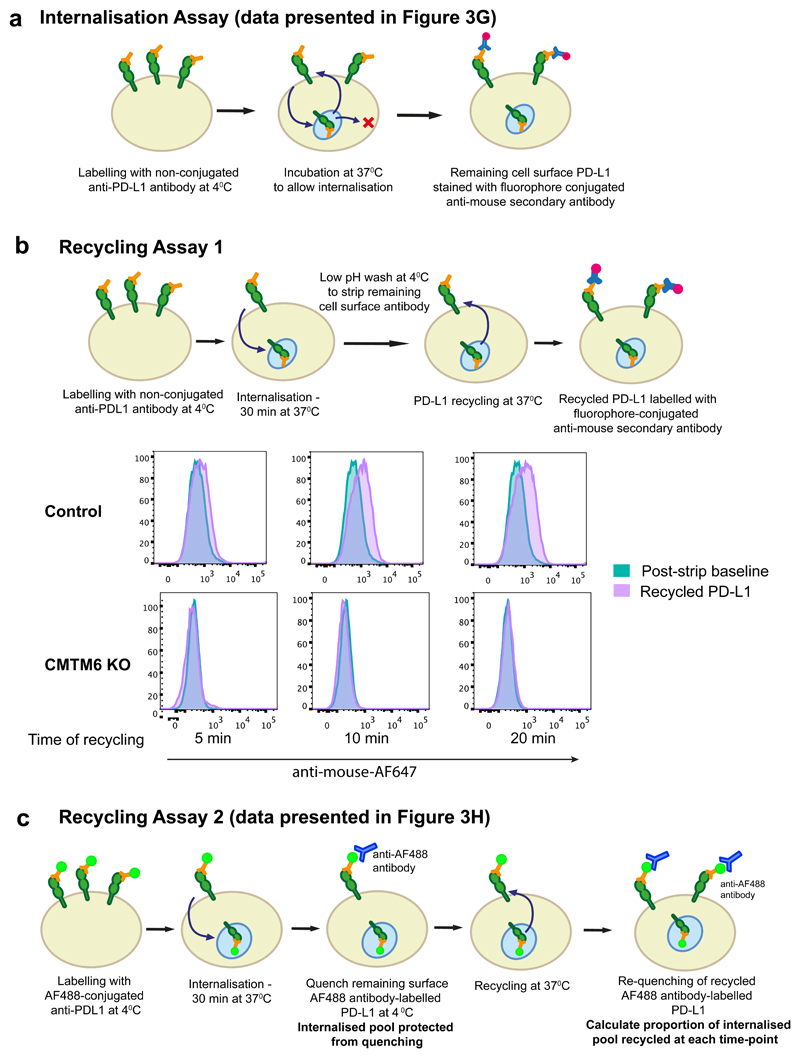
Extended Data Figure 8. PD-L1 endocytosis and recycling assays.
a. Internalisation assay (relates to data presented in Figure 3g). Cells were labelled with unconjugated PD-L1-specific antibodies at 4°C. After washing off unbound antibody, cells were incubated at 37°C to allow PD-L1 internalisation. Remaining cell surface antibody-labelled PD-L1 was detected with an AF647-conjugated anti-mouse secondary antibody and analysed by flow cytometry.
b. Recycling assay 1. Cell surface PD-L1 in IFN-γ treated CMTM6 knockout and Cas9 control WM-852 cells was labelled with an unconjugated PD-L1 specific antibody and allowed to internalise for 30 min at 37°C as described in a. Remaining cell-surface bound antibody was stripped by washing in pH 2.5 buffer and cells were either kept on ice (post-strip baseline) or re-incubated at 37°C for the indicated times. Recycled PD-L1 was detected with an AF647-conjugated anti-mouse secondary antibody.
c. Recycling assay 2 (relates to data presented in Figure 3h). Cells were labelled with an AF488-conjugated PD-L1 specific antibody, washed and incubated at 37°C to allow endocytosis. Remaining cell surface AF488 was quenched by incubation with an AF488-specific antibody for 1 h on ice prior to reincubation at 37°C to allow recycling of antibody-labelled PD-L1. Samples were split and incubated with or without an AF488-specific antibody for 1 h on ice to re-quench cell surface AF488. Recycled PD-L1 was detected by the reappearance of cell surface ‘quenchable’ AF488 signal. After normalisation for incomplete quenching at baseline by subtracting the ‘quenchable’ AF488 fluorescence at time zero, the fluorescence intensity of recycled PD-L1 at each time-point was compared to that of the total internalised PD-L1 after the endocytosis step to calculate the proportion of PD-L1 recycled.