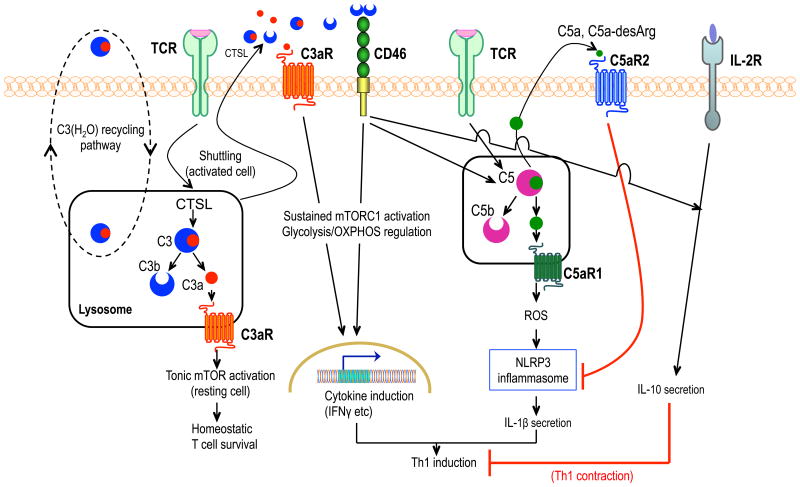
Figure 1. Complement-mediated intracellular and autocrine regulation of CD4+ T cell activation.
Under steady-state conditions, C3(H2O) is recycling between the extracellular and intracellular space. The intracellular stores of C3(H2O) constitute a source of intracellular C3a. In resting CD4+ T cells, tonic intracellular C3a generation by cathepsin L (CTSL) cleavage activates the C3a receptor (C3aR) on lysosomes, leading to low-level mTOR activation for homeostatic T-cell survival. TCR activation with CD28 co-stimulation (not shown) causes translocation of this intracellular C3 activation system to the cell surface, where C3aR and CD46 are triggered, respectively, by C3a and C3b. The ensuing C3aR- and CD46-mediated signaling events stimulate sustained mTOR complex 1 (mTORC1) activation and reprogram glycolysis and oxidative phosphorylation in ways that support TH1 (IFN-γ) responses. In activated CD4+ T cells, CD46 costimulation triggers intracellular cleavage of C5 into C5a which induces C5aR1-dependent production of reactive oxygen species, in turn activating NLRP3 inflammasome-driven IL-1β secretion that sustains TH1 induction. IL-2R signaling together with autocrine CD46 activation induces IL-10 production, initiating transition to a TH1 contraction phase. This self-regulative activity is re-inforced by cell-surface C5aR2 signaling induced by increased levels of secreted C5a (or C5a-desArg) resulting from TH1 expansion; C5aR2 downregulates C5aR1-driven NLRP3 inflammasome activation.