Figure 1.
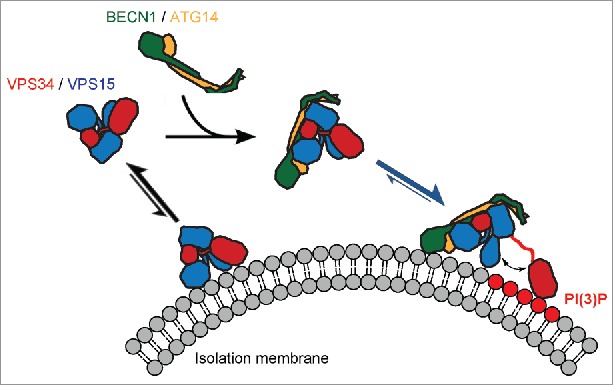
Potential mechanism for VPS34 complex assembly and activation at the membrane. Subunits are colored as follows: phosphatidylinositol 3-kinase catalytic subunit type 3 (VPS34), red; phosphoinositide 3-kinase regulatory subunit 4 (VPS15), blue; Bcl2-interacting protein 1 (BECN1), green; Beclin 1-associated autophagy-related key regulator (ATG14), orange. Phosphorylated phosphatidylinositol headgroups PtdIns(3)P are highlighted in red. Arrows indicate chemical equilibria. The scheme shows that the VPS34:VPS15 and BECN1:ATG14 subcomplexes are stable independently, however the VPS34:VPS15 subcomplex has low kinase activity and a low affinity for the membrane in the absence of the BECN1:ATG14 complex. Assembly into the 4-subunit PI3KC3-C1 complex is mediated by the HEAT and WD40 domains of the VPS15 subunit and their contacts with the BECN1:ATG14 subcomplex. This increases the affinity for the membrane and primes the complex for activation. The final step required for full activation is the dislodging of the VPS34 catalytic domain from the rest of the complex such that it engages with the membrane-bound substrate with optimal geometry and is freed from potentially inhibitory contacts with the VPS15 kinase domain.
