ABSTRACT
Purpose: We sought to estimate the prevalence of trachoma at sufficiently fine resolution to allow elimination interventions to begin, where required, in the Southern Nations, Nationalities, and Peoples’ Region (SNNPR) of Ethiopia.
Methods: We carried out cross-sectional population-based surveys in 14 rural zones. A 2-stage cluster randomized sampling technique was used. A total of 40 evaluation units (EUs) covering 110 districts (“woredas”) were surveyed from February 2013 to May 2014 as part of the Global Trachoma Mapping Project (GTMP), using the standardized GTMP training package and methodology.
Results: A total of 30,187 households were visited in 1047 kebeles (clusters). A total of 131,926 people were enumerated, with 121,397 (92.0%) consenting to examination. Of these, 65,903 (54.3%) were female. In 38 EUs (108 woredas), TF prevalence was above the 10% threshold at which the World Health Organization recommends mass drug administration with azithromycin annually for at least 3 years. The region-level age- and sex-adjusted trichiasis prevalence was 1.5%, with the highest prevalence of 6.1% found in Cheha woreda in Gurage zone. The region-level age-adjusted TF prevalence was 25.9%. The highest TF prevalence found was 48.5% in Amaro and Burji woredas. In children aged 1–9 years, TF was associated with being a younger child, living at an altitude <2500m, living in an area where the annual mean temperature was >15°C, and the use of open defecation by household members.
Conclusion: Active trachoma and trichiasis are significant public health problems in SNNPR, requiring full implementation of the SAFE strategy (surgery, antibiotics, facial cleanliness, and environmental improvement).
KEYWORDS: Ethiopia, Global Trachoma Mapping Project, prevalence, SNNPR, trachoma, trichiasis
Introduction
Trachoma is the most common infectious cause of blindness worldwide. Trachoma is thought to be endemic in at least 50 countries,1 and has been targeted for global elimination as a public health problem by the year 2020. An estimated 200 million people live in endemic areas and are at risk of developing trachoma-related blindness.2 Transmission of the causative agent, ocular strains of Chlamydia trachomatis, occurs from eye to eye via hands, clothing and bedding, or via mechanical transmission by eye-seeking flies.3–5
According to the World Health Organization (WHO) simplified trachoma grading system, trachomatous inflammation – follicular (TF) and trachomatous inflammation – intense (TI) are signs of active disease most commonly found in young children. Following repeat infections, scar tissue may form on the conjunctival surfaces of the eyelids; over time these scars can lead to in-turning of the eyelashes to the point that they abrade the surface of the globe. This is known as trachomatous trichiasis.5,6
Trachoma is thought to be endemic throughout Ethiopia, with the highest prevalences of both active and potentially blinding trachoma found anywhere in the world.7 In addition, trachoma is the country’s second most common cause of blindness overall after cataract.8 The Southern Nations, Nationalities, and Peoples’ Region (SNNPR) is a central-southern region of Ethiopia, which covers almost 110,931 km2 or 10% of the country’s land area. In 2015 it had an estimated 17.8 million inhabitants, or about a fifth of the country’s population, with more than 90% living in rural areas.9 The region is divided into 14 administrative zones, four special woredas (districts), and one city administration, with Hawassa as the regional capital.
Prior to 2013, there were limited woreda-level data regarding the distribution of trachoma in SNNPR. Trachoma intervention programs were already active in Wolaita and Gamo Gofa zones, and where eye care non-governmental organizations (NGOs) were working, trichiasis was commonly managed. However, large areas of the region had little health care coverage to provide intelligence on disease occurrence. The 2005–2006 National Survey on Blindness, Low Vision and Trachoma examined 5415 individuals in 33 kebeles (the lowest census administrative unit) throughout SNNPR and found an overall regional prevalence of active trachoma of 33.2% among children aged 1–9 years. Although this backed previous views that trachoma was a significant public health problem in the region, data at higher resolution were needed to make intervention decisions.
To guide control programs, WHO recommends district-level prevalence data are used, with the TF prevalence in children aged 1–9 years used to determine the need for the A, F and E components of the SAFE strategy (surgery for trachomatous trichiasis cases, antibiotic distribution to clear infection, facial cleanliness, and environmental improvement). In areas with a TF prevalence ≥10%, mass administration of azithromycin is recommended for at least 3 years, together with measures to encourage facial cleanliness and improve the sanitation provisions in affected communities.10,11
We carried out Global Trachoma Mapping Project (GTMP)12-supported population-based trachoma prevalence surveys covering all suspected trachoma-endemic woredas without either (1) current control programs, or (2) population-based prevalence surveys in the 10 years preceding 2013. The aims were to estimate the prevalence of TF in children aged 1–9 years, and the prevalence of trichiasis in those aged 15 years and older, to guide future interventions in the region. We also collected water, sanitation and hygiene (WASH) data to evaluate risk factors for trachoma.
Materials and methods
Although collection of district-level data is generally recommended, in areas suspected to be highly and widely endemic for trachoma, WHO accepts that baseline surveys may be powered for larger populations, in order to expedite initiation of interventions where needed.13 Our first phase of surveys was designed to estimate prevalence in each of 37 evaluation units (EUs) consisting of 1–8 contiguous, grouped woredas, with those EUs therefore corresponding to sub-zones in the local administrative hierarchy. EUs were constructed in collaboration with local epidemiologists, respecting local administrative (zonal) boundaries. In one sub-zone-level EU, we did not find trachoma to be highly endemic (see below) and extra clusters were subsequently added in each of its four constituent woredas, in a second phase of fieldwork, in order to generate woreda-level estimates of trachoma prevalence in that sub-zone. Each EU comprised a total population of up to 250,000 inhabitants. The second phase split of one EU into four EUs ultimately generated data for a total of 40 EUs, covering 110 woredas over 16 zones (Figure 1). All sampling was carried out at EU level.
Figure 1.
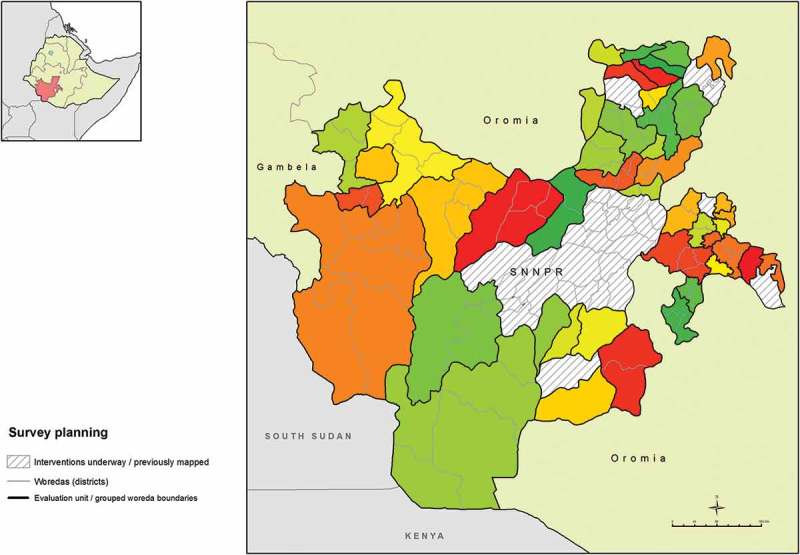
Evaluation units of grouped woredas (districts), Global Trachoma Mapping Project, Southern Nations, Nationalities, and Peoples’ Region, Ethiopia, 2013–2014.
Survey teams
Each team consisted of a trachoma grader, a data recorder and a driver. All graders and recorders had completed a standardized GTMP training course (version 1) and passed a formal examination.14 Training was conducted from 11 February 2013 to 15 February 2013. Trachoma graders were required to pass an inter-grader agreement test in the field against a GTMP-certified grader trainer. Recorders and graders were selected for training by the SNNPR Regional Health Office. Supervisors (normally ophthalmologists) who were also GTMP-certified trachoma graders or grader trainers, provided support to 3–4 teams throughout the mapping. Subsequent data processing and approval were undertaken as described elsewhere.14
Survey design and sampling
The survey used a 2-stage cluster-randomized methodology outlined in full elsewhere,14 with specific refinements for use in SNNPR. The first stage cluster unit was the kebele. Kebeles were selected using census data obtained from the regional health bureau, and were randomly chosen with probability proportional to size. Before the survey was conducted, a representative of the regional health bureau went to each selected kebele, and one developmental unit (DU; a subdivision of a kebele with approximately 30 households) was selected randomly using the lottery method. DUs which had fewer than 26 households were excluded from the sampling frame. On the day of the survey, consent was initially obtained from the village leader, and the village health extension worker or the DU head was engaged to guide the teams and to introduce the team to each household.
Sample size
As described elsewhere14 the sample size needed to estimate a 10% TF prevalence in children aged 1–9 years with an absolute precision of 3% at the 95% confidence level (and accounting for the non-independence of results in clustered sampling by using a design effect estimate from previous trachoma surveys of 2.65) was 1019 1–9-year-olds. This figure was inflated by 20% to accommodate non-response, giving an anticipated sample size of 1222 children aged 1–9 years. From the 2007 census data it was estimated that there would be on average 45 children per DU, therefore we needed to survey 26 such 30-household DU clusters in each EU.9
Data collection
All household members at sampled households were eligible for inclusion. All consenting household members aged 1 year and older were examined for TF, TI and trichiasis using the WHO simplified trachoma grading system.5,6 Data recorders also collected global positioning system (GPS) coordinates of each sampled household, and WASH data by focused interview with the household head and direct observation of sanitation facilities. WASH variables collected were consistent with the WHO/UNICEF Joint Monitoring Programme outcomes.14,15 All data were collected using Android smartphones with the Task Force LINKS software (Task Force for Global Health, Decatur, GA, USA). Data were encrypted and uploaded to a secure cloud-based server when mobile network or Wi-Fi reception was available.
Ethical considerations
Ethics clearance was obtained from SNNPR Health Bureau Ethical Review Committee, and the Ethics Review Committee of the Federal Ministry of Health of Ethiopia. Regional, zone and woreda officials were informed and provided with support letters for the study. In addition, ethics clearance was obtained from the Ethics Committee at the London School of Hygiene & Tropical Medicine (reference 6319). Verbal informed consent was obtained from the household head and electronically recorded in LINKS. All members of households found to have active trachoma were provided with a course of 1% tetracycline and instructions on its use. Those who needed further examination and treatment were referred to the nearest eye care center using a standardized referral form. Survey teams also provided general health education on limiting the spread of trachoma.
Environmental risk factors
Cluster-level climatic data thought to be related to trachoma were obtained from WorldClim variables (worldclim.org),16 at a resolution of 2.5 arc-minutes (~5 km). Altitude was collected from global positioning system (GPS) data on smartphones at the time of survey. These measures were considered to possibly associate with likelihood of C. trachomatis transmission. Variables considered for models were altitude, annual mean temperature, mean annual precipitation, and maximum temperature in the hottest month.
Data analysis
Briefly, TF estimates in the 1–9-year age range were adjusted for age in 1-year bands using the 2007 SNNPR census data.9 Trichiasis estimates were adjusted for both sex and age in 5-year bands. It was anticipated that by visiting 30 households per cluster, approximately the same number of participants would be examined per cluster. However, to account for natural variation in these numbers, clusters were equally weighted by adjusting at cluster-level and calculating the arithmetic mean of all such adjusted cluster estimates, with this mean used as the overall EU-level prevalence estimate.14 Confidence intervals (CIs) around these results were constructed by bootstrapping from these estimates and taking the 2.5th and 97.5th centiles of 10,000 bootstrap replicates.
Adjusted results and CIs were produced using R 3.0.2 (2013; The R Foundation for Statistical Computing, Vienna, Austria). Point values for climatic variables were extracted from rasters using ArcGIS 10.3 (Spatial Analyst; Environmental Systems Research Institute, Redlands, CA, USA). Risk factor analysis was carried out in Stata 10.2 (Stata Corp, College Station, TX, USA). Co-linearity was assessed using Mantel-Haenszel tests of association, but not used as an absolute exclusion criterion. A multi-level hierarchical model was used to account for clustering at kebele and household level. Univariable associations were assessed and variables were retained for the multivariable model if significant at the p < 0.05 level (Wald’s test). The full model was developed by a stepwise inclusion approach with variables retained if statistically significant at the p < 0.05 level (likelihood ratio test).
Results
The first phase of 37 sub-zone-level surveys (covering 110 woredas) was carried out from 18 February to 10 June 2013. One EU, comprising four woredas (Gorchie, Hula, Malega, Wenesho) was found to have a TF prevalence between 5% and 10%, so in a second phase, these four woredas were further mapped as four EUs at woreda-level, using the same methodology previously applied at sub-zonal level. This meant adding clusters to each constituent woreda to reach the required sample size. We undertook this second phase from 3 April 2014 to 23 May 2014.
Overall, 14 rural zones of SNNPR were included in the surveys. A total of 40 EUs covering 113 woredas were ultimately surveyed. A total of 30,187 households were visited in 1047 kebeles, with 131,926 people enumerated, and 121,397 (92.0%) consenting to examination. Of those examined, 65,903 (54.3%) were female. There was a median of four inhabitants per household (interquartile range, IQR, 3–6), and a median of one child aged 1–9 years per household (IQR 0–2). The demographic characteristics of all individuals enumerated are outlined in Table 1.
Table 1.
Demographic characteristics of individuals enumerated, Global Trachoma Mapping Project, Southern Nations, Nationalities, and Peoples’ Region, Ethiopia, 2013–2014.
| Age group, years | Sex | Examined, n (%) | Absent, n (%) | Refused, n (%) | Other, n (%) | Total, n |
|---|---|---|---|---|---|---|
| 1–9 | Male | 21,296 (96.2) | 805 (3.6) | 44 (0.2) | 4 (0.0) | 22,149 |
| Female | 20,919 (97.5) | 508 (2.4) | 29 (0.1) | 3 (0.0) | 21,459 | |
| 10–14 | Male | 8113 (88.1) | 1074 (11.7) | 21 (0.2) | 0 (0.0) | 9208 |
| Female | 7871 (90.9) | 761 (8.8) | 23 (0.3) | 2 (0.0) | 8593 | |
| ≥15 | Male | 26,085 (82.8) | 5333 (16.9) | 71 (0.2) | 7 (0.0) | 31,496 |
| Female | 37,113 (95.3) | 1767 (4.5) | 73 (0.2) | 4 (0.0) | 38,957 | |
| Total | 121,397 | 10,248 | 261 | 20 | 131,926 | |
The age-adjusted EU-level prevalence of TF in children aged 1–9 years ranged from 2.3% (95% CI 0.6–4.8%) in Geta and Gumer woredas of Gurage Zone, to 48.5% (95% CI 41.5–55.9%) in Amaro and Burji in Segen Zone. There were only two EUs which had TF prevalences below 10%; Geta and Gumer, and Gorchie woreda in Sidama. All other EUs had TF prevalences ≥10% (Table 2, Figure 2).
Table 2.
Trachomatous inflammation – follicular (TF) prevalence in children aged 1–9 years by evaluation unit, Global Trachoma Mapping Project, Southern Nations, Nationalities, and Peoples’ Region, Ethiopia, 2013–2014.
| Zone | Evaluation unit | Examined, n | TF cases, n | Unadjusted TF, % | Adjusteda TF, % (95% CI) |
|---|---|---|---|---|---|
| Hadiya | Misha, Anna Lemo, Gibe, Limo | 1093 | 498 | 45.6 | 45.0 (38.1–51.8) |
| Soro, Duna, Gombora | 1092 | 397 | 36.4 | 36.0 (28.2–44.9) | |
| Shashego, Yem, Misrak Badawacho, Mirab Badawacho | 1189 | 446 | 37.5 | 37.8 (31.6–44.8) | |
| Sidama | Dale, Shebedino | 837 | 273 | 32.6 | 30.6 (24.3–37.2) |
| Hawassa Zuria, Boroche, Wenedo Genet | 1036 | 424 | 40.9 | 39.5 (31.5–46.9) | |
| Arbegona, Bona Zuria, Bursa, Chirie | 1238 | 251 | 20.3 | 17.4 (10.1–24.2) | |
| Dara, Aleta Wendo Town, Loka Abaya, Chiko | 962 | 225 | 23.4 | 22.7 (16.7–29.3) | |
| Aroresa, Bensa | 1120 | 324 | 28.9 | 27.7 (19.8–37.0) | |
| Gorchie | 1029 | 68 | 6.6 | 6.3 (2.7–10.0) | |
| Hula | 966 | 118 | 12.2 | 10.1 (5.4–15.0) | |
| Malega | 1050 | 124 | 11.8 | 11.8 (7.2–15.3) | |
| Wenesho | 900 | 150 | 16.7 | 13.6 (9.1–18.7) | |
| Gedio | Kochere, Gedeb, Yergachefe | 1299 | 251 | 19.3 | 18.3 (13.2–22.4) |
| Bule, Wenago, Dilla Zuria | 1105 | 209 | 18.9 | 15.0 (9.4–20.1) | |
| South Omo | Basketo, South Ari, Semen Ari, Salamago, Debub Ari, North Ari | 1072 | 304 | 28.4 | 26.6 (19.5–35.9) |
| Hamer, Bena Tsemay, Dassenech, Malie, Nyngatom | 1043 | 240 | 23 | 20.1 (13.3–27.8) | |
| Sheka | Yeki, Masha Anderacha, Masha, Masha Town, Tepi Town | 863 | 110 | 12.7 | 12.0 (6.2–18.3) |
| Kafa | Chena, Gesha, Gewata, Gimbo, Saylem | 991 | 426 | 43.0 | 40.8 (32.9–48.9) |
| Bita, Cheta, Decha, Menjiwo, Tello | 1036 | 429 | 41.4 | 36.9 (29.1–44.5) | |
| Bench Maji | Bero, Shay Bench, Surma, Guraferda, Maji, Meanit Goldiiya, Meint Shasha, Debub Bench | 1115 | 398 | 35.7 | 32.0 (24.4–41.8) |
| Semen Bench, Sheko, Mizan Aman Town | 1212 | 358 | 29.5 | 28.5 (20.1–35.7) | |
| Segen | Amaro, Burji | 1301 | 658 | 50.6 | 48.5 (41.5–55.9) |
| East Gurage/Konta/Dawro | Konta, Isara, Tocha, Mareko | 927 | 330 | 35.6 | 35.6 (27.2–44.9) |
| Dawro | Gena Bosa, Loma, Tercha Town | 1030 | 303 | 29.4 | 28.1 (21.2–34.0) |
| Silti | Alicho Wuriro, Misirak Azerenert Berbere, Sankura, Mirab Azerenert Berbere, Wilbareg | 984 | 315 | 32.0 | 26.4 (18.0–36.5) |
| Dalocha, Lamfero, Selti | 1191 | 368 | 30.9 | 27.0 (18.6–33.4) | |
| Gamo Gofa | Kemba | 1158 | 350 | 30.2 | 26.4 (19.1–35.5) |
| Bonke | 1098 | 193 | 17.6 | 15.8 (9.3–24.3) | |
| Arbaminch Town | 1018 | 214 | 21.0 | 19.0 (13.5–25.5) | |
| Special | Konso | 1084 | 427 | 39.4 | 35.5 (28.2–41.9) |
| Kembata Tembaro/Halaba | Halaba Special woreda, Demboya, Kedida-Gamela | 1166 | 524 | 44.9 | 41.0 (33.8–48.5) |
| Angacha, Hadero Tunito, Kacha Bira, Doyugena, Tembaro | 1005 | 323 | 32.1 | 29.1 (24.2–35.3) | |
| Gurage | Cheha | 794 | 256 | 32.2 | 29.1 (22.4–35.4) |
| Ezha | 798 | 120 | 15.0 | 13.9 (9.8–18.9) | |
| Mihur Aklil | 784 | 234 | 29.8 | 27.6 (18.2–39.8) | |
| Kebena | 959 | 286 | 29.8 | 30.8 (24.5–38.0) | |
| Kokir | 958 | 157 | 16.4 | 15.4 (9.8–23.6) | |
| Abeshige | 958 | 155 | 16.2 | 14.7 (9.6–20.2) | |
| Sodo | 940 | 400 | 42.6 | 41.4 (31.2–49.9) | |
| Geta, Gumer | 754 | 17 | 2.3 | 2.3 (0.6–4.8) |
aAdjusted for age in 1-year age bands.
CI, confidence interval.
Figure 2.
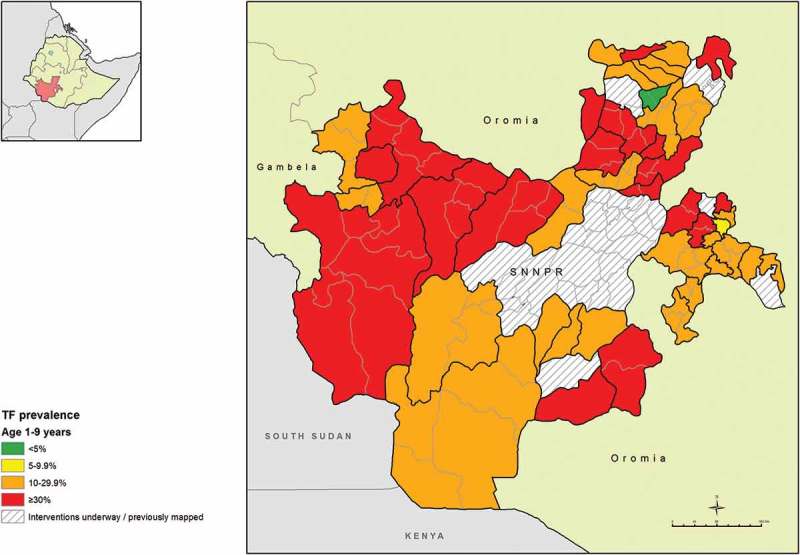
Prevalence of trachomatous inflammation – follicular (TF) in children aged 1–9 years, Global Trachoma Mapping Project, Southern Nations, Nationalities, and Peoples’ Region, Ethiopia, 2013–2014.
The age- and sex-adjusted prevalences of trichiasis in those aged 15 years and older ranged from 0.0% (95% CI 0.0–0.1%) in Hula woreda of Sidama Zone, to 6.1% (95% CI 4.4–7.8%) in Cheha in Gurage Zone. The trichiasis prevalence in those aged 15 years and older was 0.2% or less in three EUs covering six woredas in Sidama Zone; Arbergona, Bona Zuria, Bursa, Chirie, Gorchie and Hula (Table 3, Figure 3).
Table 3.
Trichiasis prevalence in those aged ≥15 years by evaluation unit, Global Trachoma Mapping Project, Southern Nations, Nationalities, and Peoples’ Region, Ethiopia, 2013–2014.
| Examined age 15 years or greater |
|||||
|---|---|---|---|---|---|
| Zone | Evaluation unit | Examined, n | Trichiasis cases, n | Unadjusted TT, % | Adjusteda TT, % 95% CI |
| Hadiya | Misha, Anna Lemo, Gibe, Limo | 1649 | 54 | 3.3 | 2.1 (1.3–2.7) |
| Soro, Duna, Gombora | 1674 | 74 | 4.4 | 2.6 (1.5–4.1) | |
| Shashego, Yem, Misrak Badawacho, Mirab Badawacho | 1615 | 70 | 4.3 | 3.0 (2.0–4.2) | |
| Sidama | Dale, Shebedino | 1686 | 34 | 2.0 | 1.6 (0.9–2.5) |
| Hawassa Zuria, Boroche, Wenedo Genet | 1592 | 27 | 1.7 | 1.1 (0.5–1.9) | |
| Arbegona, Bona Zuria, Bursa, Chirie | 1466 | 3 | 0.2 | 0.1 (0.0–0.1) | |
| Dara, Aleta Wendo Town, Loka Abaya, Chiko | 1596 | 9 | 0.6 | 0.5 (0.1–1.0) | |
| Aroresa, Bensa | 1558 | 9 | 0.6 | 0.4 (0.1–0.6) | |
| Gorchie | 1506 | 2 | 0.1 | 0.1 (0.0–0.1) | |
| Hula | 1478 | 2 | 0.1 | 0.0 (0.0–0.1) | |
| Malega | 1370 | 7 | 0.5 | 0.3 (0.0–0.5) | |
| Wenesho | 1528 | 8 | 0.5 | 0.3 (0.0–0.6) | |
| Gedio | Kochere, Gedeb, Yergachefe | 1614 | 17 | 1.1 | 0.8 (0.3–1.5) |
| Bule, Wenago, Dilla Zuria | 1456 | 22 | 1.5 | 0.7 (0.4–1.0) | |
| South Omo | Basketo, South Ari, Semen Ari, Salamago, Debub Ari, North Ari | 1205 | 13 | 1.1 | 0.6 (0.1–1.2) |
| Hamer, Bena Tsemay, Dassenech, Malie, Nyngatom | 1215 | 26 | 2.1 | 1.1 (0.5–1.5) | |
| Sheka | Yeki, Masha Anderacha, Masha, Masha Town, Tepi Town | 1987 | 21 | 1.1 | 0.7 (0.3–1.1) |
| Kafa | Chena, Gesha, Gewata, Gimbo, Saylem | 1727 | 25 | 1.4 | 1.1 (0.5–1.9) |
| Bita, Cheta, Decha, Menjiwo, Tello | 1592 | 22 | 1.4 | 0.9 (0.5–1.4) | |
| Bench Maji | Bero, Shay Bench, Surma, Guraferda,Maji, Meanit Goldiiya, Meint Shasha, Debub Bench | 1306 | 11 | 0.8 | 0.6 (0.2–1.1) |
| Semen Bench, Sheko, Mizan Aman Town | 1470 | 15 | 1.0 | 0.8 (0.3–1.3) | |
| Segen | Amaro, Burji | 1500 | 59 | 3.9 | 2.6 (1.6–3.4) |
| East Gurage/Konta/Dawro | Konta, Isara, Tocha, Mareko | 1462 | 58 | 4.0 | 2.3 (1.4–3.1) |
| Dawro | Gena Bosa, Loma, Tercha Town | 1620 | 34 | 2.1 | 1.2 (0.8–1.7) |
| Silti | Alicho Wuriro, Misirak Azerenert Berbere, Sankura, Mirab Azerenert Berbere, Wilbareg | 1434 | 27 | 1.9 | 0.8 (0.4–1.4) |
| Dalocha, Lamfero, Selti | 1468 | 52 | 3.5 | 2.0 (1.3–2.7) | |
| Gamo Gofa | Kemba | 1456 | 28 | 1.9 | 1.1 (0.5–1.8) |
| Bonke | 1481 | 27 | 1.8 | 1.2 (0.7–1.9) | |
| Arbaminch Town | 1416 | 31 | 2.2 | 0.8 (0.5–1.2) | |
| Special | Konso | 1394 | 33 | 2.4 | 1.0 (0.7–1.4) |
| Kembata Tembaro/Halaba | Halaba Special woreda, Demboya, Kedida-Gamela | 1559 | 53 | 3.4 | 2.0 (1.3–2.7) |
| Angacha, Hadero Tunito, Kacha Bira, Doyugena, Tembaro | 1640 | 77 | 4.7 | 3.1 (2.3–3.8) | |
| Gurage | Cheha | 1606 | 184 | 11.5 | 6.1 (4.4–7.8) |
| Ezha | 1895 | 97 | 5.1 | 2.2 (1.4–3.3) | |
| Mihur Aklil | 1488 | 98 | 6.6 | 4.3 (2.8–6.3) | |
| Kebena | 1546 | 125 | 8.1 | 4.5 (3.1–6.2) | |
| Kokir | 1425 | 35 | 2.5 | 1.6 (1.0–2.5) | |
| Abeshige | 1730 | 80 | 4.6 | 3.3 (2.2–4.5) | |
| Sodo | 1473 | 49 | 3.3 | 1.4 (0.7–2.0) | |
| Geta, Gumer | 1830 | 17 | 0.9 | 0.3 (0.1–0.6) | |
Adjusted for sex and age in 5-year age bands.
CI, confidence interval.
Figure 3.
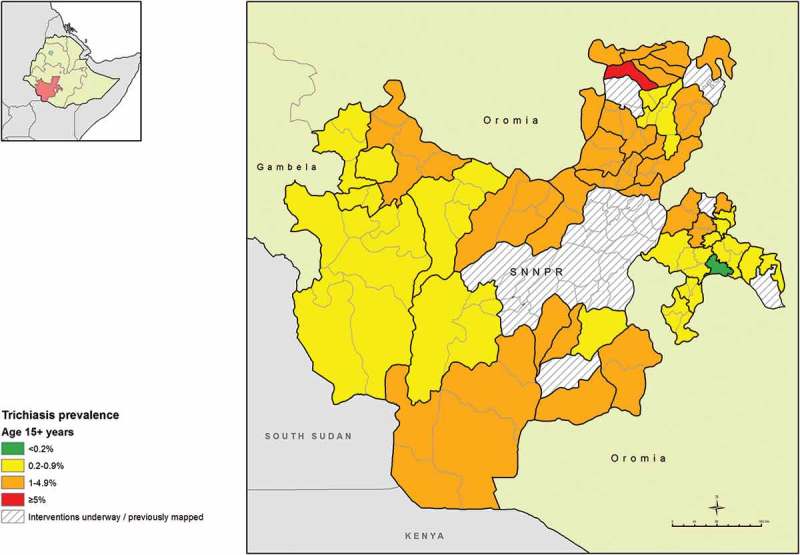
Prevalence of trichiasis in those aged ≥15 years, Global Trachoma Mapping Project, Southern Nations, Nationalities, and Peoples’ Region, Ethiopia, 2013–2014.
Multilevel clustering of TF and TI
Null models for both TF and TI adjusted for age and sex showed statistically significant clustering at EU, kebele (cluster) and household levels. For TF, the estimated standard deviation for between-EU clustering was 2.78 (standard error, SE, 1.13, p < 0.0001), for between-kebele clustering was 4.08 (SE 1.04, p < 0.0001), and for between-household clustering was 3.0 (SE 1.03, p < 0.0001). For TI, the estimated standard deviation for between-EU clustering was 2.0 (SE 1.1, p = 0.001), for between-kebele clustering was 3.17 (SE 1.06, p < 0.0001), and for between-household clustering was 3.16 (SE 1.06, p < 0.0001). For both TF and TI, clustering was strongest at kebele level, and all subsequent analyses were conducted as 2-level hierarchical regression models with adjustment for the kebele inhabited by examined individuals.
Risk factors and climatic variables
Climatic data were extracted from the raster data for all 1047 clusters. The median annual mean temperature over all clusters was 18.0°C (IQR 16.0–19.4°C), the median annual maximum temperature was 27.2°C (IQR 24.7–28.8°C), and the median annual mean precipitation was 1240mm (IQR 1124–1453mm). The median cluster-level altitude was 1984m (IQR 1769–2427m).
The full results of univariable analysis are shown in Table 4. In the full multivariable model (Table 5), factors independently associated with TF and TI in children aged 1–9 years were being a younger child, living at an altitude <2500m, and living in an area where the annual mean temperature was above 15°C. Additionally, the use of open defecation by adult household members was associated with TF, but not TI, in children aged 1–9 years.
Table 4.
Multilevel univariable analysis of factors related to trachomatous inflammation – follicular (TF) and trachomatous inflammation – intense (TI) in children aged 1–9 years, Global Trachoma Mapping Project, Southern Nations, Nationalities, and Peoples’ Region, Ethiopia, 2013–2014.
| TF |
TI |
||||
|---|---|---|---|---|---|
| Variable | Examined, n | % | Odds ratioa (95% CI) | % | Odds ratioa (95% CI) |
| Individual | |||||
| Age, years | |||||
| 1–4 | 17,649 | 36.4 | 2.66 (2.53–2.81) | 5.3 | 2.40 (2.16–2.68) |
| 5–9 | 25,960 | 20.5 | 1 (reference) | 2.4 | 1 (reference) |
| Sex | |||||
| Male | 22,149 | 26.9 | 1 (reference) | 3.5 | 1 (reference) |
| Female | 21,459 | 27.0 | 1.01 (0.97–1.06) | 3.7 | 0.89 (0.77–1.02) |
| Household | |||||
| Children aged 1–9 years in household, n | |||||
| 1–3 | 34,061 | 27.2 | 1 (reference) | 3.7 | 1 (reference) |
| ≥4 | 9547 | 26.0 | 1.02 (0.96–1.09) | 3.3 | 0.89 (0.78–1.02) |
| Inhabitants in household, n | |||||
| 1–7 | 36,477 | 27.8 | 1 (reference) | 3.6 | 1 (reference) |
| ≥8 | 7131 | 22.6 | 0.91 (0.85–1.02) | 3.2 | 0.97 (0.82–1.14) |
| Time to main source of drinking water, minutes | |||||
| <30 | 20,378 | 26.7 | 1 (reference) | 3.8 | 1 (reference) |
| ≥30 | 23,230 | 27.2 | 0.97 (0.90–1.05) | 3.4 | 0.94 (0.81–1.09) |
| Time to main source of water used for face-washing, minutes | |||||
| All washing at source | 112 | 28.6 | 1.02 (0.43–2.39) | 6.3 | 2.81 (0.69–11.27) |
| <30 | 21,996 | 26.4 | 1 (reference) | 3.8 | 1 (reference) |
| ≥30 | 21,500 | 27.4 | 1.00 (0.93–1.08) | 3.3 | 0.90 (0.77–1.04) |
| Surface waterb used as drinking source | |||||
| Yes | 5296 | 30.5 | 1.19 (1.03–1.38) | 4.6 | 1.21 (0.95–1.55) |
| No | 38,312 | 26.4 | 1 (reference) | 3.4 | 1 (reference) |
| Surface waterb used for face-washing | |||||
| Yes | 8018 | 28.4 | 1.11 (0.99–1.24) | 4.5 | 1.29 (1.06–1.58) |
| No | 35,590 | 26.6 | 1 (reference) | 3.4 | 1 (reference) |
| Latrine typec | |||||
| Improved latrine | 2367 | 23.8 | 1 (reference) | 3.0 | 1 (reference) |
| Unimproved latrine | 33,937 | 26.3 | 1.07 (0.93–1.22) | 3.5 | 1.10 (0.83–1.46) |
| Open defecation (no facilities, bush, or field) | 7304 | 31.1 | 1.18 (1.02–1.37) | 4.1 | 1.28 (0.95–1.76) |
| Kebele (cluster) | |||||
| Altituded, m above sea level | |||||
| <2500 | 34,696 | 31.5 | 1 (reference) | 4.3 | 1 (reference) |
| ≥2500 | 8883 | 8.9 | 0.21 (0.17–0.25) | 0.8 | 0.18 (0.13–0.24) |
| Mean annual precipitatione, mm | |||||
| <1000 | 6403 | 35.4 | 2.01 (1.54–2.63) | 4.7 | 1.66 (1.23–2.24) |
| 1000–1499 | 27,968 | 24.5 | 1 (reference) | 3.2 | 1 (reference) |
| ≥1500 | 9237 | 28.5 | 1.41 (1.13–1.77) | 4.0 | 1.44 (1.11–1.85) |
| Mean annual temperaturee, ◦C | |||||
| <15 | 7724 | 7.0 | 0.10 (0.08–0.13) | 0.8 | 0.16 (0.11–0.23) |
| ≥15 | 35,884 | 31.2 | 1 (reference) | 4.2 | 1 (reference) |
| Maximum temperature of warmest monthe, °C | |||||
| <25 | 11,150 | 12.3 | 0.18 (0.15-0.22) | 1.2 | 0.23 (0.18-0.31) |
| ≥25 | 32,458 | 32.0 | 1 (reference) | 4.4 | 1 (reference) |
aMultilevel univariable random effects regression accounting for clustering at kebele-level, with 95% CI; p-value from Wald’s test; variables significant at the p < 0.10 level are shown in bold.
bRiver, dam, lake, canal.
cWHO/UNICEF Joint Monitoring Programme improved sanitation definitions; open defecation displayed separately.
dEstimate from GPS coordinates of household measured on smartphones at the time of survey.
eEstimate from GPS coordinates of household, extracted at kebele(cluster)-level from Bioclim rasters (Worldclim.org).
CI, confidence interval; WHO, World Health Organization; GPS, global positioning system.
Table 5.
Multivariable multi-level risk factor model for the outcomes trachomatous inflammation – follicular (TF) and trachomatous inflammation – intense (TI) in children aged 1–9 years, Global Trachoma Mapping Project, Southern Nations, Nationalities, and Peoples’ Region, Ethiopia, 2013–2014.
| Variable |
TF model |
TI model |
||
|---|---|---|---|---|
| Odds ratioa (95% CI) | P-valueb | Odds ratioa (95% CI) | P-valueb | |
| Age, years | <0.0001 | <0.0001 | ||
| 1–4 | 2.65 (2.52–2.79) | 2.38 (2.13–2.65) | ||
| 5–9 | 1 (reference) | 1 (reference) | ||
| Mean annual temperaturec, °C | <0.0001 | 0.001 | ||
| <15 | 0.16 (0.11–0.23) | 0.42 (0.25–0.69) | ||
| ≥15 | 1 (reference) | 1 (reference) | ||
| Altituded, metres above sea level | <0.0001 | <0.0001 | ||
| <2500 | 1 (reference) | 1 (reference) | ||
| ≥2500 | 0.50 (0.40–0.70) | 0.31 (0.20–0.49) | ||
| Latrine typee | 0.0311 | 0.1163f | ||
| Improved latrine | 1 (reference) | 1 (reference) | ||
| Unimproved latrine | 1.10 (0.96–1.26) | 1.17 (0.88–1.56) | ||
| Open defecation (no facilities, bush, or field) | 1.19 (1.02–1.38) | 1.33 (0.97–1.82) | ||
aMultilevel multivariable random effects regression accounting for clustering at kebele-level. Sex was included in the model a priori, but was not statistically significant for either TF or TI (p < 0.05 level, likelihood ratio test).
bLikelihood ratio test.
cEstimate from GPS coordinates of household, extracted at kebele-level from Bioclim rasters (Worldclim.org).
dEstimate from GPS coordinates of household measured on smartphones at the time of survey.
eWHO/UNICEF Joint Monitoring Programme improved sanitation definitions; open defecation displayed separately.
fNot included in final TI model, but presented for illustrative purposes.
CI, confidence interval; WHO, World Health Organization; GPS, global positioning system.
Discussion
Trachoma is clearly a major public health problem in SNNPR. A total of 38 of 40 EUs surveyed had TF prevalences above the 10% threshold at which WHO recommends population-level distribution of antibiotics for 3 years before re-survey. One of 37 sub-zone-sized EUs had a TF prevalence between 5.0% and 9.9% in the first phase, and was re-mapped at woreda level in the second phase; three of four constituent woredas had TF prevalences above 10%, while the fourth had a TF prevalence between 5.0% and 9.9%. The final EU (composed of two woredas) had a TF prevalence of 2.3%, below the elimination threshold for TF. This means that in SNNPR, 108 woredas require mass drug administration (MDA) with azithromycin for at least 3 years, plus implementation of the F and E components of the SAFE strategy, before impact surveys. Overall, 15 of the 38 EUs (48 woredas) had TF prevalences above 30% and will require MDA for at least 5 years before impact surveys are needed.
The reason for the density of hyper-endemic woredas in SNNPR is unclear, and the risk factor analysis did not help much to generate hypotheses. Younger children have been associated with higher odds of trachoma in many previous studies.17–19 In addition, open defecation is thought to increase infection transmission by providing an ecological niche for eye-seeking flies known to carry C. trachomatis.20,21 Trachoma has been associated with altitude before,21 but the finding in these surveys of a lower odds of trachoma at high (≥2500m) altitudes is difficult to explain. It has previously been suggested that at higher altitudes, lower temperatures decrease fly activity,22 but why this would cause an effect independent of temperature at low altitude is unclear. It is possible that lower altitudes in SNNPR provide a climate where people are more likely to settle, and so human population density may be an important association in these areas.
The prevalence of trichiasis throughout SNNPR was high. In 37 of 40 EUs (104 woredas) the prevalence was higher than the elimination prevalence target (set by WHO) of <0.2% in the population 15 years of age and older.23 It is likely that without robust interventions being rapidly put in place, many people will go blind in SNNPR in the coming years as they wait for surgery. This warrants the development and maintenance of a strong program that will address the backlog of patients with trichiasis. To illustrate the magnitude of the undertaking required, 184 cases of trichiasis were identified in the EU covering Cheha woreda alone, giving an estimated trichiasis prevalence of 6.1% of the adult population there. Cheha has been known as a trachoma hotspot for many years, and eye care NGOs have been targeting trichiasis surgery outreach programs there since as early as 2006, yet even now, the prevalence is still among the highest trichiasis prevalence estimates ever reported. The reason that Cheha should have such a large trichiasis burden is unclear, but research may be helpful to guide the regional program as it seeks to recruit, train and deploy trichiasis surgeons to address it, and to understand the barriers that people in these areas have to seeking healthcare.
Our use of EUs generally comprising multiple woredas allowed us to more rapidly complete baseline mapping of SNNPR than if each woreda had been approached as an individual EU. However, as interventions decrease TF prevalence, it is likely that impact surveys will need to be carried out at higher resolution, in other words, at woreda-level. This will require a significant increase in funding to support the trachoma elimination effort in SNNPR.
Active and potentially blinding trachoma are highly prevalent throughout SNNPR, and in 108 woredas the disease presents a significant public health problem. Political support, coordination and funding for trachoma elimination are all urgently required.
Appendix
The Global Trachoma Mapping Project Investigators are: Agatha Aboe (1,11), Liknaw Adamu (4), Wondu Alemayehu (4,5), Menbere Alemu (4), Neal D. E. Alexander (9), Berhanu Bero (4), Simon J. Brooker (1,6), Simon Bush (7,8), Brian K. Chu (2,9), Paul Courtright (1,3,4,7,11), Michael Dejene (3), Paul M. Emerson (1,6,7), Rebecca M. Flueckiger (2), Allen Foster (1,7), Solomon Gadisa (4), Katherine Gass (6,9), Teshome Gebre (4), Zelalem Habtamu (4), Danny Haddad (1,6,7,8), Erik Harvey (1,6,10), Dominic Haslam (8), Khumbo Kalua (5), Amir B. Kello (4,5), Jonathan D. King (6,10,11), Richard Le Mesurier (4,7), Susan Lewallen (4,11), Thomas M. Lietman (10), Chad MacArthur (6,11), Colin Macleod (3,9), Silvio P. Mariotti (7,11), Anna Massey (8), Els Mathieu (6,11), Siobhain McCullagh (8), Addis Mekasha (4), Tom Millar (4,8), Caleb Mpyet (3,5), Beatriz Muñoz (6,9), Jeremiah Ngondi (1,3,6,11), Stephanie Ogden (6), Alex Pavluck (2,4,10), Joseph Pearce (10), Serge Resnikoff (1), Virginia Sarah (4), Boubacar Sarr (5), Alemayehu Sisay (4), Jennifer L. Smith (11), Anthony W. Solomon (1,2,3,4,5,6,7,8,9,10,11), Jo Thomson (4); Sheila K. West (1,10,11), Rebecca Willis (2,9).
Key: (1) Advisory Committee, (2) Information Technology, Geographical Information Systems, and Data Processing, (3) Epidemiological Support, (4) Ethiopia Pilot Team, (5) Master Grader Trainers, (6) Methodologies Working Group, (7) Prioritisation Working Group, (8) Proposal Development, Finances and Logistics, (9) Statistics and Data Analysis, (10) Tools Working Group, (11) Training Working Group.
Funding Statement
This study was principally funded by the Global Trachoma Mapping Project (GTMP) grant from the United Kingdom’s Department for International Development (ARIES: 203145) to Sightsavers, which led a consortium of non-governmental organizations and academic institutions to support ministries of health to complete baseline trachoma mapping worldwide. The GTMP was also funded by the United States Agency for International Development (USAID) through the ENVISION project implemented by RTI International under cooperative agreement number AID-OAA-A-11-00048, and the END in Asia project implemented by FHI360 under cooperative agreement number OAA-A-10-00051. A committee established in March 2012 to examine issues surrounding completion of global trachoma mapping was initially funded by a grant from Pfizer to the International Trachoma Initiative. AWS was a Wellcome Trust Intermediate Clinical Fellow (098521) at the London School of Hygiene & Tropical Medicine. None of the funders had any role in project design, in project implementation or analysis or interpretation of data, in the decisions on where, how or when to publish in the peer-reviewed press, or in preparation of the manuscript.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Funding
This study was principally funded by the Global Trachoma Mapping Project (GTMP) grant from the United Kingdom’s Department for International Development (ARIES: 203145) to Sightsavers, which led a consortium of non-governmental organizations and academic institutions to support ministries of health to complete baseline trachoma mapping worldwide. The GTMP was also funded by the United States Agency for International Development (USAID) through the ENVISION project implemented by RTI International under cooperative agreement number AID-OAA-A-11-00048, and the END in Asia project implemented by FHI360 under cooperative agreement number OAA-A-10-00051. A committee established in March 2012 to examine issues surrounding completion of global trachoma mapping was initially funded by a grant from Pfizer to the International Trachoma Initiative. AWS was a Wellcome Trust Intermediate Clinical Fellow (098521) at the London School of Hygiene & Tropical Medicine. None of the funders had any role in project design, in project implementation or analysis or interpretation of data, in the decisions on where, how or when to publish in the peer-reviewed press, or in preparation of the manuscript.
References
- 1. World Health Organization WHO Alliance for the Global Elimination of Blinding Trachoma by the year 2020. Progress report on elimination of trachoma, 2013. Wkly Epidemiol Rec 2014;89:421–428. [PubMed] [Google Scholar]
- 2. World Health Organization Trachoma: Fact sheet. Geneva: World Health Organization, 2016. [Google Scholar]
- 3. Emerson PM, Lindsay SW, Alexander N, et al. Role of flies and provision of latrines in trachoma control: cluster-randomised controlled trial. Lancet 2004;363:1093–1098. [DOI] [PubMed] [Google Scholar]
- 4. West SK, Congdon N, Katala S, et al. Facial cleanliness and risk of trachoma in families. Arch Ophthalmol 1991;109:855–857. [DOI] [PubMed] [Google Scholar]
- 5. Solomon AW, Peeling RW, Foster A, et al Diagnosis and assessment of trachoma. Clin Microbiol Rev 2004;17:982–1011, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thylefors B, Dawson CR, Jones BR, et al. A simple system for the assessment of trachoma and its complications. Bull World Health Organ 1987;65:477–483. [PMC free article] [PubMed] [Google Scholar]
- 7. Berhane Y, Alemayehu W, Bejiga A.. National Survey on Blindness, Low Vision and Trachoma in Ethiopia. Addis Ababa: Federal Democratic Republic of Ethiopia, 2006. [Google Scholar]
- 8. Bourne RRA, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health 2013;1:e339–349. [DOI] [PubMed] [Google Scholar]
- 9. Central Statistical Agency Federal Democratic Republic of Ethiopia Population Census. Addis Ababa: Federal Democratic Republic of Ethiopia Population Census Commission, 2007. [Google Scholar]
- 10. Solomon A, Zondervan M, Kuper H, et al. Trachoma control: a guide for programme managers. Geneva: World Health Organization, 2006. [Google Scholar]
- 11. Emerson PM, Burton M, Solomon AW, et al. The SAFE strategy for trachoma control: using operational research for policy, planning and implementation. Bull World Health Organ 2006;84:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Solomon AW, Kurylo E.. The Global Trachoma Mapping Project. Community Eye Health J 2014;27:18. [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization Report of the 3rd Global Scientific meeting on trachoma, Johns Hopkins University, Baltimore, MD, 19–20 July 2010. Geneva: World Health Organization, 2010. [Google Scholar]
- 14. Solomon AW, Pavluck A, Courtright P, et al. The Global Trachoma Mapping Project: methodology of a 34-country population-based study. Ophthalmic Epidemiol 2015;22:214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. UNICEF and WHO Progress on Sanitation and Drinking Water: 2015 Update and MDG Assessment. Geneva: World Health Organization, 2015. [Google Scholar]
- 16. Hijmans RJ, Cameron SE, Parra JL, et al. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol Int J Clim 2005;25:1965–1978. [Google Scholar]
- 17. Cajas-Monson LC, Mkocha H, Muñoz B, et al. Risk factors for ocular infection with Chlamydia trachomatis in children 6 months following mass treatment in Tanzania. PLoS Negl Trop Dis 2011;5:e978. doi: 10.1371/journal.pntd.0000978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edwards T, Harding-Esch EM, Hailu G, et al. Risk factors for active trachoma and Chlamydia trachomatis infection in rural Ethiopia after mass treatment with azithromycin. Trop Med Int Health 2008;13:556–565. [DOI] [PubMed] [Google Scholar]
- 19. Last AR, Burr SE, Weiss HA, et al. Risk factors for active trachoma and ocular Chlamydia trachomatis infection in treatment-naïve trachoma-hyperendemic communities of the Bijagós Archipelago, Guinea Bissau. PLoS Negl Trop Dis 2014;8:e2900. doi: 10.1371/journal.pntd.0002900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Golovaty I, Jones L, Gelaye B, et al. Access to water source, latrine facilities and other risk factors of active trachoma in Ankober, Ethiopia PLoS One 2009;4:e6702. doi: 10.1371/journal.pone.0006702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramesh A, Kovats S, Haslam D, et al. The impact of climatic risk factors on the prevalence, distribution, and severity of acute and chronic trachoma PLoS Negl Trop Dis 2013;7:e2513. doi: 10.1371/journal.pntd.0002513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Emerson PM, Bailey RL.. Trachoma and fly control. Community Eye Health J 1999;12:57. [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization Validation of elimination of trachoma as a public health problem (WHO/HTM/NTD/2016.8). Geneva: World Health Organization, 2016. [Google Scholar]


