Figure 4. Western blot analysis of HERV-proteins in the mitochondrial fraction of U87RETO glioblastoma cells upon etoposide incubation with 5 μg/ml for 10 days.
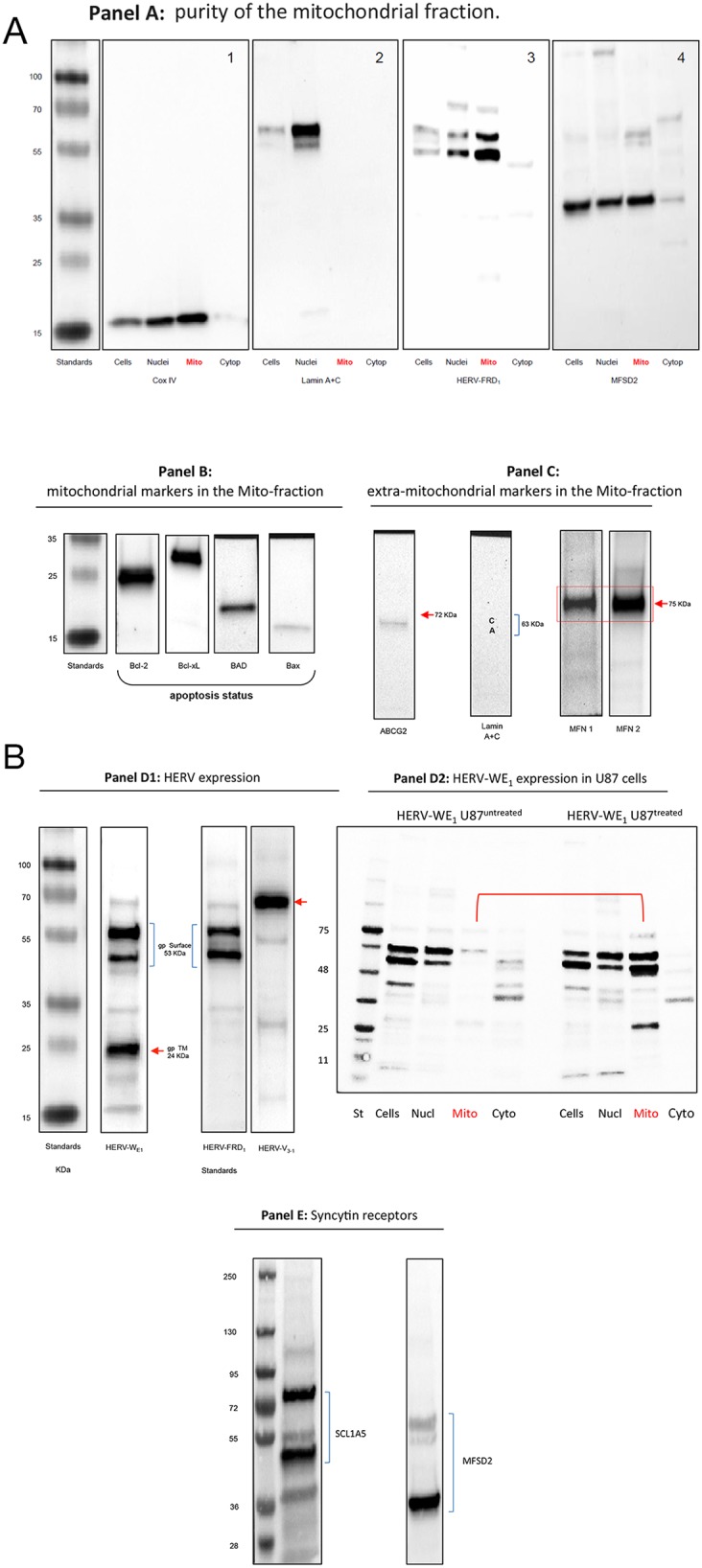
Panel A: controls. A1: Mitochondrial Cox IV was detected in whole cell extracts, nuclear and mitochondrial fractions. A2: lamin A & C markers were detected only in nuclear fractions indicating the absence of nuclear contaminations in the mitochondrial fraction except for cytoplasmic preparations. Panel A3 reflects the content of syncytin 2 (HERV-FRD1) in all sub-cellular preparations with remarkable expression in the mitochondrial fraction of U87RETO cells. Panel A4: detection of the receptor for syncytin 2, MFSD2 in subcellular fractions except for cytoplasmic preparations. Panel B minor expression of pro-apoptotic proteins like BAD and BAX in U87RETO cells. In contrast, anti-apoptotic proteins like Bcl-2 and Bcl-xL were found strongly expressed. (For comparison of cytoplasmic vs. whole cell distributions of BAX in untreated vs. treated cells, showing no difference upon etoposide-incubation, see Supplementary Data.) Panel C: controls: isolated mitochondria with mitochondrial markers MFN1 and MFN2 (positive control) almost without extra-mitochondrial membrane proteins like ABCG2 and lamin A+C (negative controls). Panel D1: expression of different HERV proteins in the mitochondrial fraction of U87RETO cells. For syncytin 1 (HERV-WE1, upper bands) the 53 kDa surface protein and an additional splicing variant below is shown. In addition, the 24 kDa band reflects the transmembrane protein of syncytin 1. For syncytin 2 the 24 kDa protein was not detectable. HERV-V3-1 was detectable as the expected single band at 32 kDa. Panel D2: analysis of intracellular distribution of syncytin 1 in untreated U87RETO cells (control, left lanes) vs. treated U87RETO cells after incubation with 5 μg/ml etoposide for 10 days. HERV proteins were found enhanced in the mitochondrial fraction upon etoposide stress. Comparable results were obtained for syncytin 2, see Supplementary Data. Panel E: after incubating U87RETO cells with 5 μg/ml etoposide for 10 days, receptors for syncytin 1 and 2 were also clearly detectable in mitochondrial fraction (see also panel A4). For SCL1A5, the endogenous protein (53 kDa) and various glycosylated forms were detected. The receptor for syncytin 2, MFSD2, was also detectable as a single band. The figure is representative of at least n = 3 independent experiments.
