Table 1. Summary of reported direct AMPK activators.
| Compounds | Chemical structure | Binding subunit | Cell line | Effect time | Effect concentration | Reference |
|---|---|---|---|---|---|---|
| Salicylate | 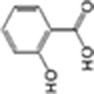 |
β | A549 | 48 h | 103.64 ± 4.59 μM | [74] |
| A-769662 | 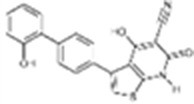 |
β | A549 | 48 h | 10 μM | [13] |
| Compound 991 | 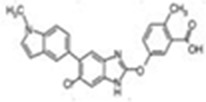 |
α β |
AML | 48 h | 100 μM | [65] [63] |
| MT 63–78 | 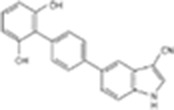 |
β | PC3 | 48 h | 25-50 μM | [75] |
| PT-1 | 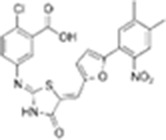 |
α γ |
HelaHEK293 | 6 h1 h | 20-40 μM100 μM | [66][67] |
| OSU-53 | 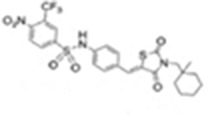 |
α | MDA-MB-231/MDA-MB-468 | 72 h24-72 h | 5 μM5-10 μM | [71][69] |
| Compound-13 | 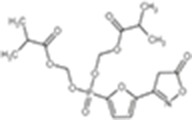 |
α | Mouse hepatocytesGastric epithelial cells | 3 h24 h | 10-100 μM10 μM | [72][73] |
| CNX-012-570 | Not shown | β | HepG2/C2C12, 3T3L1 | 2-4 h | 0.3 μM | [76] |
