Abstract
The NLRP3 inflammasome is a critical component of the innate immune system and can be activated in response to microbial and endogenous danger signals. Activation of the NLRP3 inflammasome results in caspase-1–dependent secretion of the proinflammatory cytokines IL-1β and IL-18. Gain-of-function missense mutations in NLRP3 result in a group of autoinflammatory diseases collectively known as the cryopyrin-associated periodic syndromes (CAPS). CAPS patients have traditionally been successfully treated with therapeutics targeting the IL-1 pathway; however, there are a number of identified CAPS patients who show only a partial response to IL-1 blockade. In this issue of the JCI, McGeough et al. demonstrated that TNF-α, in addition to IL-1β, plays an important role in promoting NLRP3 inflammasomopathies.
Cryopyrin-associated periodic syndromes
Gain-of-function mutations in NLRP3 are associated with autoinflammatory conditions known as the cryopyrin-associated periodic syndromes (CAPS) and include familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and neonatal-onset multisystem inflammatory disease (NOMID) (1–3). CAPS are rare disorders with a prevalence of one to two cases per million people in the United States and Europe (4). CAPS patients typically present with recurrent fevers, arthralgias, conjunctivitis, and neutrophilic urticaria (4). The symptoms associated with FCAS are usually the least severe and are precipitated by exposure to cold, while the symptoms of MWS are intermediate and frequently associated with hearing loss. Patients with NOMID, on the other hand, exhibit symptoms associated with MWS and, additionally, develop severe, deforming arthropathy and CNS inflammation, with more chronic and continuous symptoms. Both germline and somatic mutations in NLRP3 can cause CAPS, and over 130 mutations, mainly in the central nucleotide-binding domain (NBD) of NLRP3, have been identified to date. IL-1 blockade is the mainstay in the treatment of CAPS patients and is extremely effective for most of them. However, in clinical practice, an increasing number of CAPS patients respond partially to treatment with IL-1 blockers. These failures in clinical treatments suggest that additional inflammatory pathways may contribute to CAPS pathology in specific cases.
The NLRP3 inflammasome
Activation of the NLRP3 inflammasome is a two-step process (5). While priming, the first step, is mediated through a variety of cytokine or pattern recognition receptors and results in the NF-κB–mediated transcriptional upregulation of NLRP3 and pro–IL-1β activation, the second step occurs in response to specific NLRP3 agonists, such as silica, monosodium urate (MSU), nigericin, or ATP. In a pathway that is not fully understood, these two signals result in the assembly and activation of the inflammasome complex composed of NLRP3, the adaptor apoptosis-associated speck-like protein containing a CARD domain (ASC), and pro–caspase-1. Common cellular signals required for NLRP3 activation include potassium efflux, calcium influx, and mitochondrial dysfunction, with the generation of mitochondrial R0S (5). Activation of the NLRP3 inflammasome culminates in the autocatalysis of pro–caspase-1 into the active cysteine protease caspase-1, which leads to the cleavage of pro–IL-1β and pro–IL-18 into their mature secreted forms (Figure 1). NLRP3 inflammasome–mediated caspase-1 activation can also lead to a gasdermin D–driven pyroptotic cell death (6, 7). Importantly, the activation signal is not required in patients with CAPS, and challenging CAPS macrophages with LPS alone can lead to the robust processing and secretion of IL-1β (8). Although the identification of gain-of-function mutations in NLRP3 in CAPS patients was the first indication of the role of NLRP3 in human disease, aberrant activation of the NLRP3 inflammasome is now implicated in a variety of chronic inflammatory and metabolic disorders including atherosclerosis, Alzheimer’s disease, and type 2 diabetes mellitus (9). Blockade of the IL-1 pathway has been actively investigated as a potential therapeutic approach for diseases in which the NLRP3 inflammasome plays a detrimental role.
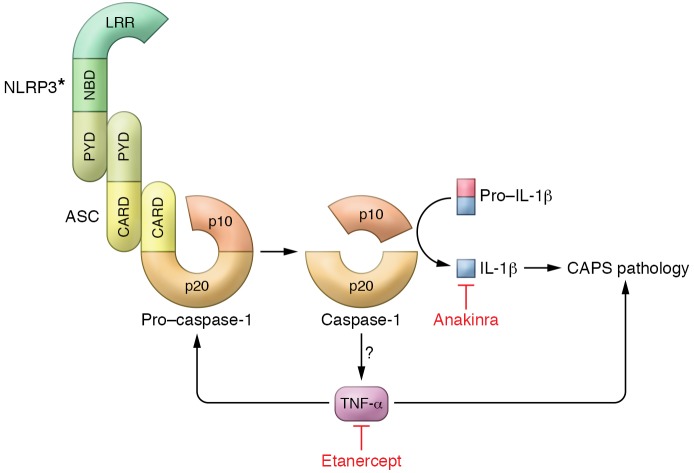
Figure 1. Model of IL-1β– and TNF-α–driven pathology in CAPS.
Gain-of-function mutations in NLRP3 (asterisk denotes the mutant protein) result in the formation and activation of the NLRP3 inflammasome, composed of NLRP3, the adapter protein ASC, and caspase-1, in response to a proinflammatory stimulus (priming). This results in the autocatalytic activation of the cysteine protease pro–caspase-1 to generate two subunits, p20 and p10. Active caspase-1 then processes pro–IL-1β into its mature secreted form. Activation of the NLRP3 inflammasome in Nlrp3-mutant knockin CAPS mice results in the production of TNF-α through an unknown mechanism. This TNF-α contributes to CAPS disease pathology and also transcriptionally regulates caspase-1 and pro–IL-1β expression. Anakinra and etanercept can diminish CAPS pathology through blockade of IL-1β and TNF-α, respectively. CARD, caspase activation and recruitment domain; LRR, leucine-rich repeat domain; PYD, pyrin domain.
A role for TNF-α in CAPS pathogenesis
Brydges et al. (10) and Meng et al. (11) both used different Nlrp3-mutant knockin mouse strains to model human CAPS. Brydges et al. generated Nlrp3-mutant knockin strains expressing NLRP3A350V and NLRP3L351P to model human MWS and FCAS mutations, respectively (10). Meng et al. generated an Nlrp3-mutant mouse that expressed NLRP3R258W to study the human MWS mutation (11). Both studies demonstrated a central role for myeloid cell production of inflammasome-dependent IL-1β in driving disease pathology in the Nlrp3-mutant knockin mice. Additionally, Meng et al. showed that the Nlrp3 gene–targeted mice also developed Th17-dependent inflammatory skin disease (11). Using myeloid-restricted Nlrp3L351P-mutant knockin mice crossed with IL-1β– and IL-18–deficient mice (Nlrp3L351P Il1b–/– Il18–/–), McGeough et al. further evaluated the contribution of IL-1β and IL-18 to the pathogenesis of CAPS disease (12). Not only did they observe a partial phenotypic rescue, they also showed that, despite the absence of the classic NLRP3 inflammasome–dependent cytokines IL-1β and IL-18, by 6 months of age, Nlrp3L351P Il1b–/– Il18–/– mice had evidence of persistent systemic inflammation with elevated white blood cell counts and splenomegaly with neutrophilic infiltrates. Importantly, this chronic inflammation was not seen in Nlrp3L351P mice crossed with caspase-1/11 knockouts (Nlrp3L351P Casp1/11–/–), suggesting that the mutated NLRP3 and caspase-1 and/or caspase-11 were responsible for driving an inflammatory response independently of IL-1β and IL-18. When challenged with a sublethal dose of LPS, Nlrp3L351P Il1b–/– Il18–/– mice succumbed rapidly, while the Nlrp3L351P Casp1/11–/– mice did not. There was also a significant and specific early elevation in serum levels of TNF-α, but not IL-6, IL-1α, IL-17, or KC, in Nlrp3L351P Il1b–/– Il18–/– mice as compared with levels in Nlrp3L351P Casp1/11–/– mice, suggesting that in Nlrp3L351P mice, the NLRP3 inflammasome and caspase-1 and/or caspase-11 were responsible for driving TNF-α production in response to LPS (Figure 1). To confirm the relevance of these mouse data to patients, McGeough et al. evaluated serum samples from NOMID patients before and after treatment with the recombinant IL-1 receptor antagonist anakinra (12). Although CAPS symptoms and chronic inflammation in the NOMID patients were reduced after anakinra therapy, the drug also led to elevated serum TNF-α levels, consistent with the elevated TNF-α levels observed in the Nlrp3L351P Il1b–/– Il18–/– mice following LPS challenge.
As Nlrp3L351P LysM-Cre mice die perinatally in the absence of IL-1β/IL-18 blockade, McGeough et al. used Nlrp3A350V LysM-Cre mice, which display a less severe phenotype, to investigate the role of TNF-α in NLRP3 inflammasome–driven pathology (12). Treating Nlrp3A350VLysM-Cre mice with the TNF-α inhibitor etanercept resulted in both a strikingly improved growth rate as well as increased survival of mice. Consistent with this finding, crossing Nlrp3A350V LysM-Cre mice with TNF-α–deficient mice (Nlrp3A350V Tnf–/–) resulted in the animals surviving to adulthood, with no evidence of the skin erythema and splenomegaly that were observed in Nlrp3A350V LysM-Cre mice. Intriguingly, Nlrp3A350V Tnf–/– mice also had reduced serum IL-1β and IL-18 levels compared with Nlrp3A350V LysM-Cre mice, suggesting that TNF-α may play a regulatory role upstream of the NLRP3 inflammasome–mediated production of IL-1β and IL-18. In light of these data, the role of TNF-α in NLRP3 inflammasome priming and activation was analyzed in vitro. The mRNA expression of Casp1 and Il1b was reduced in bone marrow–derived DCs from Nlrp3A350V Tnf–/– mice, suggesting that the mechanism by which TNF modifies the release of IL-1β and IL-18 may be through the upregulation of the NLRP3 inflammasome components and pro–IL-1β (Figure 1). These findings are consistent with the earlier work of Nakamura et al. showing that treatment of Nlrp3R258W-mutant knockin mice with a TNF-α–blocking monoclonal antibody rescued neonatal Nlrp3R258W mice from the development of inflammatory skin disease and splenomegaly (13). TNF-α blockade in Nlrp3R258W mice also inhibited the production of IL-1β. However, treatment of adult Nlrp3R258W mice with TNF-α–blocking monoclonal antibody failed to abrogate ongoing inflammatory skin disease (13).
Conclusions and future directions
This study by McGeough and colleagues demonstrated that in CAPS mouse strains expressing mutant Nlrp3, a caspase-1 and/or caspase-11 inflammatory phenotype is present and is independent of the traditional NLRP3 inflammasome–dependent cytokines IL-1β and IL-18 (12). They further showed that blockade of TNF-α using etanercept or genetic knockouts abrogated both disease pathology and IL-1β production in Nlrp3A350V mice. Although clearly dependent on the presence of the NLRP3 inflammasome, the cellular source of the TNF-α and the mechanism by which it is generated remain unclear. Since the Nlrp3L351P Casp1/11–/– mice used in this study were deficient in both caspase-1 and caspase-11, it is possible that caspase-11–mediated noncanonical inflammasome activation may participate in driving the production of TNF-α. It remains to be determined whether the association of TNF-α with NLRP3 inflammasome activation is specific to the gain-of-function mutations or is more generally applicable to WT NLRP3 inflammasome activation, which would have broad implications for a variety of NLRP3 inflammasome–mediated chronic inflammatory and metabolic diseases that may be targeted by IL-1 blockade.
Nlrp3A350VTnf–/– mice also unexpectedly showed impaired IL-1β and IL-18 production, suggesting that TNF-α plays a regulatory role in activation of the NLRP3 inflammasome. As an explanation for similar observations, Nakamura et al. postulated that mast cells locally produced TNF-α in response to commensal microbes and that this TNF-α induced the production of IL-1β in mast cells with CAPS-associated NLRP3 mutations, resulting in an amplification of the inflammatory response (13). Further work to elucidate the role of the commensal microbiota in triggering disease in CAPS patients, and whether this involves the induction of TNF-α, is certainly warranted.
Importantly, the blockade of IL-1 is extremely effective in the treatment of a majority of CAPS patients (14–16), while the blockade of TNF-α has only shown modest benefit (17–19). Despite this general efficacy, some CAPS patients only partially responded to IL-1 blockade, and as we now know, the treatment of NOMID patients with anakinra is associated with an elevation of serum TNF-α, suggesting a more complex underlying pathophysiology (12). While the role of TNF-α in the pathogenesis of CAPS requires further study, the work by McGeough et al. suggests that the subset of CAPS patients who fail to respond completely to IL-1 blockade may benefit from TNF-α blockade. While additional immunomodulation may be beneficial to address the symptoms of these patients, this combination therapy may introduce further issues, as a previous study of rheumatoid arthritis patients treated with a combination of anakinra and etanercept had an increased risk of infectious complications (20). However, while the specifics of the implementation of this combination therapy in CAPS patients may require more consideration, the association of TNF with pathology in CAPS, and potentially with other NLRP3-mediated inflammatory diseases, opens a door to a new class of therapeutics to target this globally important pathway.
Acknowledgments
We thank Suzanne Cassel (Cedars-Sinai Medical Center, Los Angeles, CA, USA) for helpful discussions and Eric Elliott (University of Iowa, Io¡wa City, IA, USA) for assistance with artwork. NIH grant R01 AI118719 (to FSS) and a grant from the Harry J. Lloyd Charitable Trust (to FSS) supported this work.
Version 1. 11/13/2017
Electronic publication
Version 2. 12/01/2017
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information: J Clin Invest. 2017;127(12):4235–4237. https://doi.org/10.1172/JCI98322.
See the related article at TNF regulates transcription of NLRP3 inflammasome components and inflammatory molecules in cryopyrinopathies.
Contributor Information
Balaji Banoth, Email: Balaji.Banoth@cshs.org.
Fayyaz S. Sutterwala, Email: Fayyaz.Sutterwala@cshs.org.
References
- 1.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29(3):301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aksentijevich I, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46(12):3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldmann J, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71(1):198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldbach-Mansky R, Kastner DL. Autoinflammation: the prominent role of IL-1 in monogenic autoinflammatory diseases and implications for common illnesses. J Allergy Clin Immunol. 2009;124(6):1141–1149. doi: 10.1016/j.jaci.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kayagaki N, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 7.Shi J, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 8.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20(3):319–325. doi: 10.1016/S1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 9.Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev. 2011;243(1):152–162. doi: 10.1111/j.1600-065X.2011.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brydges SD, et al. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity. 2009;30(6):875–887. doi: 10.1016/j.immuni.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30(6):860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGeough MD, et al. TNF regulates transcription of NLRP3 inflammasome components and inflammatory molecules in cryopyrinopathies. J Clin Invest. 2017;127(12):4488–4497. doi: 10.1172/JCI90699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura Y, Franchi L, Kambe N, Meng G, Strober W, Núñez G. Critical role for mast cells in interleukin-1β-driven skin inflammation associated with an activating mutation in the nlrp3 protein. Immunity. 2012;37(1):85–95. doi: 10.1016/j.immuni.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldbach-Mansky R, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355(6):581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman HM, et al. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet. 2004;364(9447):1779–1785. doi: 10.1016/S0140-6736(04)17401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lachmann HJ, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360(23):2416–2425. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 17.Ebrahimi-Fakhari D, Wahlster L, Mackensen F, Blank N. Clinical manifestations and longterm followup of a patient with CINCA/NOMID syndrome. J Rheumatol. 2010;37(10):2196–2197. doi: 10.3899/jrheum.100290. [DOI] [PubMed] [Google Scholar]
- 18.Federico G, Rigante D, Pugliese AL, Ranno O, Catania S, Stabile A. Etanercept induces improvement of arthropathy in chronic infantile neurological cutaneous articular (CINCA) syndrome. Scand J Rheumatol. 2003;32(5):312–314. doi: 10.1080/03009740310003974. [DOI] [PubMed] [Google Scholar]
- 19.Matsubara T, et al. A severe case of chronic infantile neurologic, cutaneous, articular syndrome treated with biologic agents. Arthritis Rheum. 2006;54(7):2314–2320. doi: 10.1002/art.21965. [DOI] [PubMed] [Google Scholar]
- 20.Genovese MC, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50(5):1412–1419. doi: 10.1002/art.20221. [DOI] [PubMed] [Google Scholar]