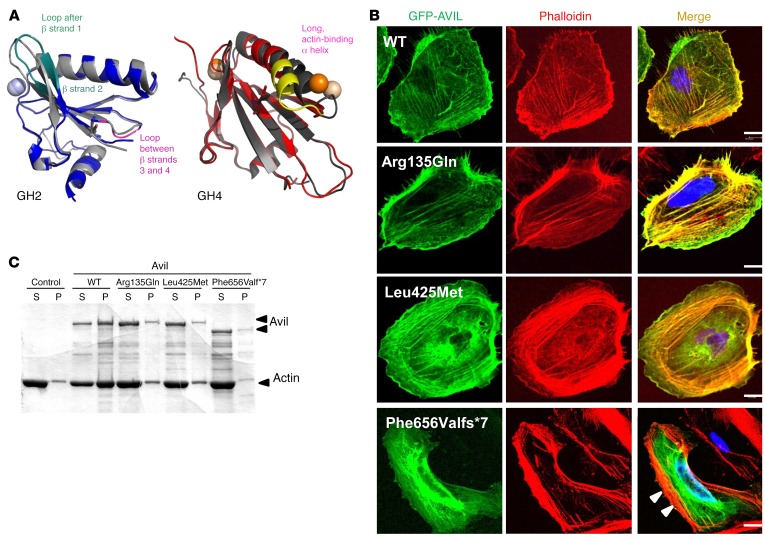
Figure 3. Mutations in AVIL affect podocyte cytoskeleton architecture and inhibit the actin-bundling function of AVIL.
(A) Locations of mutations in gelsolin homology domains of advillin and their effect on conformational flexibility. Overlay of averaged structures of WT (dark gray) and mutated GH2 (blue) and GH4 (red) domains from MD studies of human AVIL (PDB references: 1RGI and 1H1V). Averaging was carried out over the whole scaled MD trajectory. A homology model for hAvil-1 was built using gelsolin as a template based on the crystal structures of actin-bound gelsolin 1H1V4 (S4–S6 or GH4–GH6 domains) (57) and 1RGI (S1–S3 or GH1–GH3 domains) (58). The models built served as starting structures for all MD studies. Structural elements with increased conformational flexibility are highlighted in teal and magenta for the GH2 domain and in yellow for the GH4 domain. Mutated residues are depicted with sticks, and spheres denote calcium ions. (B) Colocalization of AVIL with F-actin using cDNA clones representing WT and mutations detected in individuals with SRNS (Table 1). Human podocytes were transfected with GFP-tagged WT or the mutant AVIL (Arg135Gln, Leu425Met, or Phe656Valfs*7) and were stained for F-actin with phalloidin (red). Colocalization of AVIL and phalloidin-labeled F-actin resulted in yellow fluorescence. Note that podocytes with overexpression of the truncating mutant Phe656Valfs*7 lost the costaining pattern with phalloidin-labeled F-actin at the cell periphery (white arrowheads). Cell nuclei were stained with DAPI (blue). Scale bars: 10 μm. (C) For the actin-bundling assay, WT or mutant (Arg135Gln, Leu425Met, or Phe656Valfs*7) Avil GST proteins (1 μM) were incubated with F-actin. To determine actin bundling, samples were centrifuged at 10,000 g for 15 minutes, and actin distribution in supernatant (S) and pellet (P) fractions was analyzed by 10% SDS-PAGE and GelCode Blue staining. Control refers to F-actin filaments in the absence of Avil GST protein.