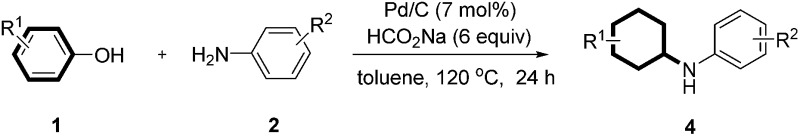
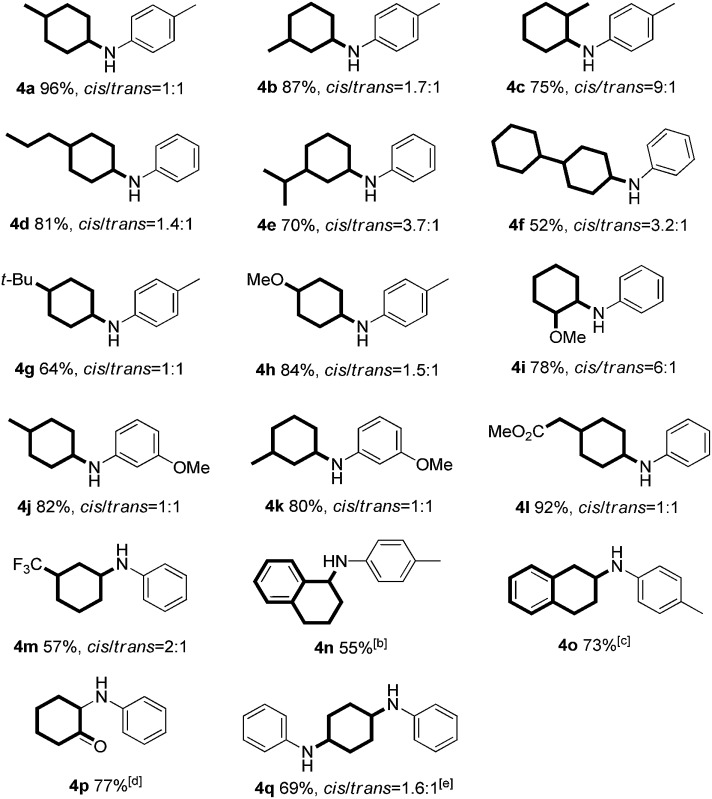
Table 3. Reaction of various substituted phenols and aniline derivatives a .
|
|
aReaction conditions: 1 (0.2 mmol), 2 (0.24 mmol), Pd/C (7 mol%), sodium formate (6 equiv.) and toluene (0.8 mL) at 120 °C for 24 h under an argon atmosphere; isolated yield and the ratio of cis/trans isomers was determined by crude 1H NMR analysis.
bNaphthalen-1-ol was used as substrate.
cNaphthalen-2-ol was used as substrate.
dCatechol was used as substrate.
eHydroquinone was used as substrate with 2 equiv. aniline.
