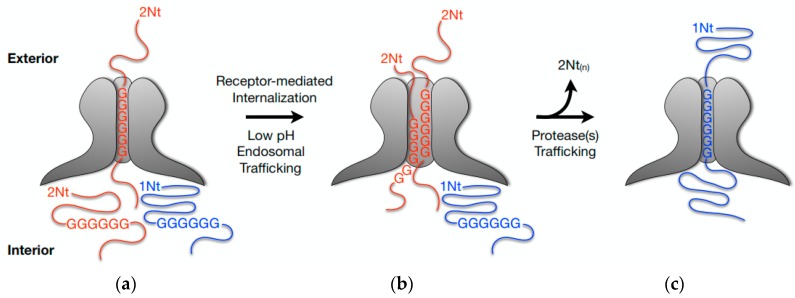
Figure 2.
Model of the sequential externalization of VPs N-terminal sequences at the capsid pore during MVM entry: (a) In the extracellular virion the N-terminal sequence of VP2 subunits (VP2-Nt or 2Nt) are externalized through the capsid channel at the five-fold axis without impairing infectivity. One subunit anchored by the poly-Gly track is illustrated; (b) Upon receptor-mediated internalization and low pH exposure inside the endosome, internal VP2 N-termini access the base of the pore enlarging the functional diameter of the channel as they become extruded to the capsid exterior; (c) Most de novo exposed VP2 N-termini are cleaved-off from the capsid surface. The incoming virion traffics to downstream endosomal compartment(s), with presumed lower protease pools, where the VP1 N-terminus (or 1Nt) is entirely externalized becoming anchored by its poly-Gly track, as the channel returns to a narrow configuration. 1Nt (or VP1-Nt), the VP1-unique N-terminal sequence (143 amino acids); 2Nt (or VP2-Nt), the VP2 N-terminal sequence (27 amino acids); GGGG, poly-Glycine track. Based on references [82,91].