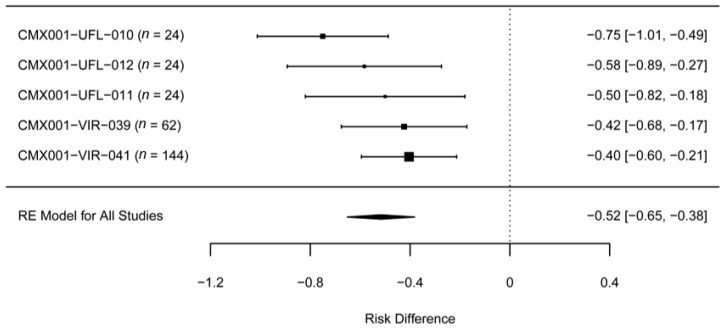
Figure 1.
Forest plot of BCV efficacy data in the rabbitpox model. The vertical dotted line is a reference for no risk difference (i.e., no drug effect). All studies included equal numbers of male and female rabbits. Studies CMX001-UFL-010, -011, and -012 were conducted at the University of Florida [14]. In these studies, BCV was administered orally at 20 mg/kg beginning at the first observation of lesions. In Study UFL-010, animals received a total of three doses of 20 mg/kg, one dose every 48 h (20/20/20 mg/kg q48h), while in Studies UFL-011 and -012, the animals received 1 or 2 doses, respectively. Studies CMX001-VIR-039 and -041 were conducted at Battelle [15,16]. In Study VIR-039, BCV was administered at the first observation of lesions. Animals received 5/5/5, 20/5/5, or 20/20/20 mg/kg q48h. In the pivotal study VIR-041, BCV was administered at the first observation of fever, or was delayed by 24, 48, or 72 h after fever. Animals received 20/5/5 mg/kg q48h. Treatment effect was estimated by calculating the risk difference for each study. A pooled estimate of the risk difference across all studies was estimated using meta-analysis methodology. For calculation of the risk difference in each study (right column (95% CI)), the treatment groups were combined into one group of BCV-treated animals, regardless of the level of efficacy in each dose group. The pooled estimate of the risk difference is 52% (95% CI: 65% to 38%), meaning the average mortality in BCV-treated animals is 52% lower on an absolute basis. Removing BCV doses above those used in Study CMX001-VIR-041 (20/5/5 mg/kg q48 h) from the analysis (i.e., 20/20/20 mg/kg q48h and 20/20 mg/kg q48h) shifts the pooled estimate of the risk difference to 41% (95% CI: 55% to 27%).