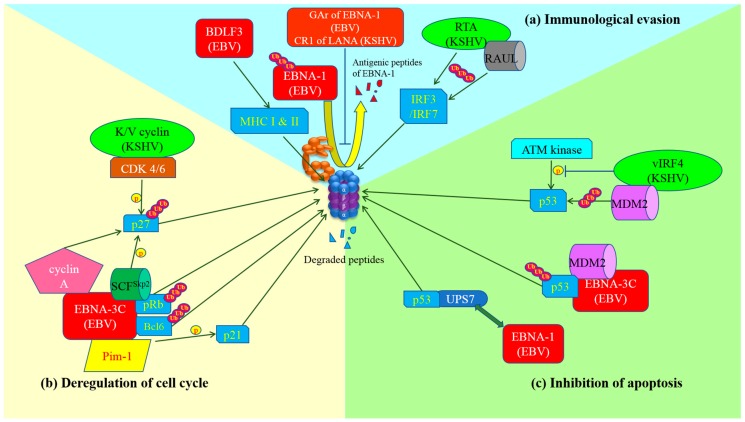
Figure 1.
Schematic diagram of exploitation of ubiquitin-proteasome system by gamma-herpesviruses and the development of cancer hallmarks. (a) Immunological evasion: GAr domain of Epstein-Barr virus (EBV) nuclear antigen (EBNA)-1 or Kaposi’s sarcoma-associated herpesvirus (KSHV) central repeat (CR)1 of latency-associated nuclear antigen (LANA)inhibits proteasome so as to prevent the proteolysis of EBNA-1 and the production of its antigenic peptides for major histocompatibility complex (MHC) class I presentation. BDLF3 promotes internalization and proteasomal degradation of MHC molecules. As a result, cytotoxic T lymphocytes (CTLs) are not able to detect and kill the latent viruses-infected cells. Replication and transcription activator (RTA) (KSHV) itself or through stabilization of RTA-associated ubiquitin ligase (RAUL) facilitates the ubiquitination and proteasomal degradation of interferon regulatory factor (IRF)3 and IRF7, which are important for innate immunity; (b) Deregulation of cell cycle: EBNA-3C can stably interact with pRb and recruit SKP1-Cul1-F-box protein (SCF)Skp2 E3-ubiquitin ligase to promote degradation of pRb. Thus, E2F is released and activates the transcription of cyclin-dependent kinases for cell cycle progression. Moreover, EBNA-3C also physically interacts with and degrades Bcl-6 through ubiquitin-specific protease (UPS) but the ligase that facilitates the ubiquitination is still under investigation. EBNA-3C interacts with Pim-1 which enhances the phosphorylation of p21WAF1 and promotes proteasomal degradation of p21WAF1. The association of SCFSkp2 with EBNA-3C or cyclin-dependent kinase (CDK)4/6 with K/V cyclin (KSHV) increases phosphorylation and proteasomal degradation of p27KIP1. Additionally, stabilization of cyclin A by EBNA-3C also promotes degradation of p27KIP1 through UPS. In summary, gamma-herpesviruses possess multiple mechanisms to assist the infected cells to bypass cell cycle checkpoints for proliferation; (c) Inhibition of apoptosis: EBNA-1 displaces p53 in the interaction with ubiquitin-specific protease 7 (USP7), resulting in destabilization of p53 and its degradation by proteasome. On the other hand, MDM2 E3-ubiquitin ligase is recruited and stabilized by EBNA-3C or vIRF4 (KSHV), leading to ubiquitination and proteasomal degradation of p53. Viral interferon regulatory factor (vIRF)4 also inhibits the phosphorylation of p53 by ataxia-telangiectasia mutated (ATM) kinase upon DNA damage response, causing destabilization and proteasomal degradation of p53.